Ischaemic Optic Neuropathy in Southeast Asia A different pattern of disease
James F. Cullen, MD, FRCS, FRCSEd
Ischaemic optic neuropathy (ION) is the commonest adult optic neuropathy encountered worldwide and likely to increase in incidence with our ageing population. The average age of onset in a report of the more common, non-arteritic variety from Singapore was 61 years.1
The aetiology of the condition is defective circulation to the optic nerve which is supplied from the ophthalmic artery, the first intracranial branch of the internal carotid artery. It was not, however, until the optic nerve blood supply was fully evaluated by Hayreh in the late 1960s that the condition could be properly understood (Figure 1).

Figure 1. Blood supply of the optic nerve after Hayreh
CRA/CAR: Central retinal artery. CRV: Central retinal vein.
PCA: Posterior ciliary artery. CZ: Circle of Zinn. D: Dura.
A: Arachnoid. P: Pia.
ION is classified as follows:
(a) Arteritic: usually associated with giant cell arteritis (GCA)
(b) Non-arteritic: by far the commonest variety seen in this region and associated with underlying vascular disease
The condition is clinically divided into anterior (AION) and posterior (PION).
Anterior ION usually involves the visible optic nerve head with optic disc swelling due to involvement of the ciliary branches of the ophthalmic artery. Posterior ION involves the pial blood supply to the more posterior optic nerve. This variety being much less common and difficult to diagnose.
Arteritic ION occurs in classical and occult forms. The latter variety presents with acute and often complete visual loss unassociated with typical systemic symptoms of GCA.
The optic nerve head is divided into laminar, prelaminar, and surface layers. The latter alone being supplied from the surface retinal vessels, whereas the other two layers along with the retrolaminar area are supplied via the posterior ciliary circulation (Figure 1).
Arteritic Ischaemic Optic Neuropathy
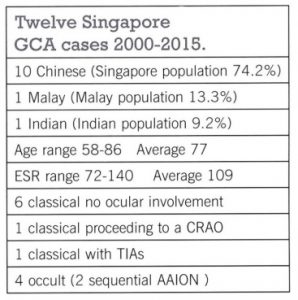
The commonest aetiology for this variety of ION is GCA which, however, is a disease seldom encountered in Southeast Asia, only 12 biopsy-proven cases being reported from Singapore over 15 years (Table 1). The condition must still be recognised because failure to diagnose when it occurs can lead to blindness. GCA affects medium-sized and large blood vessels especially the external carotid artery and its branches. In particular, the superficial temporal arteries where the symptoms of pain and tenderness therein gave the original name of “Temporal Arteritis” to the condition. The optic nerve involvement is invariably in the optic nerve head presenting with acute visual loss as an anterior ischaemic process, i.e. arteritic anterior ischaemic optic neuropathy (AAION). The nerve is infarcted and appears pale, white and swollen due to occlusion of its ciliary blood supply, and thus recovery cannot be expected (Figure 2). If untreated, second eye involvement will occur in one third of cases within 24 hours and further thirds within one week and one month.
Table 1. Details of 12 biopsy-proven cases of GCA seen over a 15-year period in Singapore with racial population percentage in parenthesis.
AAION occurs in classical and occult forms.3 This occult form usually presents with unilateral and, sometimes even, with bilateral acute visual loss in the absence of typical systemic symptoms of GCA such as scalp tenderness and jaw claudication.

Figure 2. Photograph of an optic nerve head showing a pale, swollen, infarcted disc with normal retinal circulation as seen in arteritic anterior ischemic optic neuropathy associated with giant cell arteritis.
The pathology of the condition has been demonstrated in a number of publications. The first from the United Kingdom being reported in 1958 showing the optic nerve ciliary circulation involved by GCA along with a patent central retinal artery.5 The erythrocyte sedimentation rate (ESR) in this patient was 12 mm. Figure 3 shows the pathology of another of our cases with a clearly infarcted optic nerve head along and a well-demarcated retrolaminar lesion due to occlusion of the supplying posterior ciliary vessels (this patient’s ESR was 92 mm).
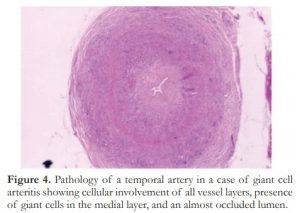
Figure 3. Pathology of arteritic anterior ischemic optic neuropathy due to giant cell arteritis showing infarction of the optic nerve head and retrolaminar area. Big red arrow points to the line of demarcation between infarcted and normal nerve. Occluded ciliary vessels are also seen (small, red arrow).
Immediate treatment with corticosteroids will prevent sequential visual involvement. Biopsy of the superficial temporal artery (Figure 4) is essential for diagnosis and should be performed within 48 hours of presentation. The ESR and C-reactive protein, preferably the former, are elevated and are used to monitor progress. Contrary to popular belief, GCA can remain active for many years or even indefinitely.3 Eventually the disc may become cupped and mistaken for glaucoma.

Figure 4. Pathology of a temporal artery in a case of giant cell arteritis showing cellular involvement of all vessel layers, presence of giant cells in the medial layer, and an almost occluded lumen.
It should be noted that GCA mainly affects branches of the external carotid artery. On the contrary, the ophthalmic artery is a branch of the internal carotid artery and why this vessel, and in particular its tiny ciliary arteries branches, are specifically affected in an AAION has not been explained. Other branches of the internal carotid artery, such as the central retinal artery and the vessels supplying the brain, are not involved.6
Apart from GCA, other even rarer causes of an AAION include systemic vasculitides such as systemic lupus erythematosus, periarteritis nodosa, and Takayasu’s disease.
Arteritic Posterior Ischaemic Optic Neuropathy
Posterior ION is rare and essentially a diagnosis of exclusion.7 No pathological proof of the condition associated with GCA has been reported.
Non-arteritic Ischaemic Optic Neuropathy
When Dr. KR from Manila visited my clinic in Singapore in 2010 for one working week, she saw 57 neuro-ophthalmology cases with me.8 The commonest optic neuropathy encountered being non-arteritic anterior ischaemic optic neuropathy (NA-AION). This condition is, in fact, the commonest cause of permanent but, fortunately, incomplete visual loss in the elderly population of Southeast Asia. It is usually associated with underlying vascular disease, especially diabetes and hypertension. It should be noted that the correct acronym for this common condition is
NA-AION as used by Hayreh in his textbook and by most non-American authors.9 The condition is invariably of the anterior variety, the posterior type again being a diagnosis of exclusion.
The clinical picture of NA-AION is of a middle-aged or elderly patient presenting with acute, painless visual loss often noticed on awakening in the morning and usually involving the lower visual field. Visual acuity may be normal but a relative afferent pupillary defect (RAPD) is present. Most importantly, the optic disc is hyperaemic and oedematous (Figure 5). Of note, the optic disc is hyperaemic even though this is an ischaemic process and thus often mistaken for optic neuritis. Visual field examination is essential for correct diagnosis; an inferior or lower altitudinal defect being typical of NA-AION (Figure 6). The disc swelling resolves over 3-6 weeks and eventually becomes flat and pale; often a yellowish pallor is seen (Figure 7). The condition occurs most often in a small, so-called “disc-at-risk” and is due to an incomplete occlusion of the ciliary blood supply to the disc, whereas in arteritic AION, the circulation is completely shut off and the disc infarcted.

Figure 5. Optic disc photograph showing a hyperaemic oedematous optic disc as seen in acute non-arteritic anterior ischemic optic neuropathy. Flame-shaped haemorrhages are often seen in this condition.
The microcirculation in the optic nerve head has been further evaluated by Olver et al. who demonstrated superior and inferior anastomotic channels connecting the medial and lateral posterior ciliary arteries (Figure 8).10 The superior anastomosis, presumably the less efficient one, is thus the watershed area in the event of perfusion failure. Its involvement explains the common inferior visual field defect encountered in acute NA-AION. These tiny vessels are gradually occluded by inflammation and the acute visual loss may be precipitated by a fall in perfusion or hydrostatic pressure as occurs on awakening or arising in the morning.
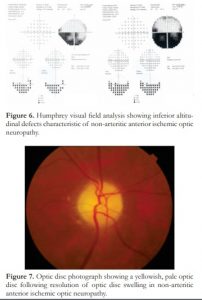
Figure 6. Humphrey visual field analysis showing inferior altitudinal defects characteristic of non-arteritic anterior ischemic optic neuropathy.
Figure 7. Optic disc photograph showing a yellowish, pale optic disc following resolution of optic disc swelling in non-arteritic anterior ischemic optic neuropathy.
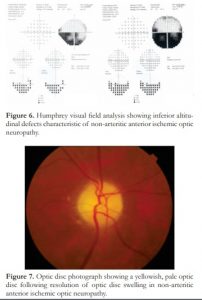
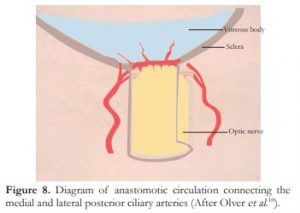
Figure 8. Diagram of anastomotic circulation connecting the medial and lateral posterior ciliary arteries (After Olver et al.).
The disc post-NA-AION (Figure 7) does not become cupped as opposed to that sometimes seen with the arteritic variety. Functional recovery in the involved visual field is not to be expected but the condition is stable at six months.11
There is no proven effective specific treatment but obviously any underlying vascular disease needs to be controlled and hypertensive therapy should be taken only early in the day. Lowering of the intraocular pressure may help as this facilitates perfusion in the nerve head and has, at least, a logical therapeutic basis. Because the underlying pathological process is one of nonperfusion and not embolic, thrombotic, or inflammatory, there is no place for treatment designed for such conditions.
Sequential eye involvement occurs in 14.5% of cases over five years12 and ipsilateral recurrence is rarely encountered.
A recent editorial in Ophthalmology co-authored by WM Hoyt has suggested that NA-AION is not an ischaemic process but due to vitreous separation from the optic disc.13 Letters to the editor following the publication from Dr. Hayreh and the current author refute this suggestion and there has been no further report from the authors on the matter.14,15
There is no convincing pathological report in respect of NA-AION unlike that for the arteritic variety as shown earlier. An illustration in Cogan’s book (Figure 9) depicting a retrolaminar infarct in the optic nerve could be an instance of NA-AION but there is no clinical report in the text to support this possibility.
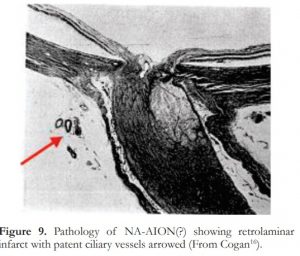
Figure 9. Pathology of NA-AION(?) showing retrolaminar infarct with patent ciliary vessels arrowed (From Cogan).
Non-arteritic Posterior Ischaemic Optic Neuropathy
In the rare circumstance of a diagnosis of PION being proposed, the non-arteritic variety, NA-PION, is the more likely in Southeast Asia. Because the more posterior optic nerve is supplied from its surrounding pial vessels, the central portion of the nerve in the event of perfusion deficiency would become the watershed area. The macular axons from the retina make their way to the centre thus a central scotoma (Figure 10) will be seen in the event of perfusion compromise in the more posterior optic nerve. The nerve head will be normal in the acute phase becoming pale in 4-6 weeks as seen in retrobulbar optic neuritis. NA-PION however must not be diagnosed without the exclusion of a compressive lesion or other causes by adequate neuroimaging.

Figure 10. Humphrey visual field analysis (top) and Goldmann visual field (bottom) demonstrating cecocentral scotoma in posterior ischemic optic neuropathy.
REFERENCES
1. Cullen JF, Cheung SH. Non-arteritic Anterior Ischaemic Optic Neuropathy (NA-AION): outcome for visual acuity and visual field defects, the Singapore scene 2. Singapore Med J. 2012;53:88-90.
2. Hayreh SS. Blood supply of the optic nerve head and its role in optic atrophy glaucoma and oedema of the optic disc. Br J Ophthalmol. 1969;53:721-748.
3. Cullen JF, Chan Joy, Wong CF, Chew WC. Giant Cell (Temporal) Arteritis in Singapore, an occult case and the Rationale of Treatment. Singapore Med J. 2010;51:73-77
4. Cullen JF, Jonasson F, Cullen JF, Elton RA. Temporal Arteritis. Scot Med J. 1979;24:111-7.
5. Cullen JF. Temporal arteritis 50 years on. Br J Ophthalmol. 2009;93:833-4.
6. Butt Z, Cullen JF, Mutlukan E. Pattern of arterial involvement of the head, neck, and eyes in giant cell arteritis: three case reports. Br J Ophthalmol. 1991;75:368-371.
7. Hayreh SS. Posterior ischaemic optic neuropathy: clinical features, pathogenesis and management. Eye. 2004;18:1188-1206.
8. Reyes KB, Cullen JF. A week in Neuro-ophthalmology: the Singapore scene. Philipp J Ophthalmol. 2010;35:70-2.
9. Hayreh SS. Ischaemic optic neuropathies. 2011. Heidelberg Springer.
10. Olver JM, Spalton DJ, Mc Cartney ACE. Microvascular Study of the Retrolaminar Optic Nerve in Man: the Possible Significance in Anterior Ischaemic Optic Neuropathy. Eye. 1990;4:7-24.
11. Hayreh SS, Zimmerman MB. Non-arteritic anterior ischemic optic neuropathy: Natural history of visual outcome. Ophthalmology. 2008;115:298-305.
12. Newman NJ, Schirer RA, Langerby P et al. The fellow eye in NAION: Report from the ION decompression trial follow up study. Am J Ophthalmol. 2002;134:312-328.
13. Parsa CF, Hoyt WF. Non Arteritic Anterior Ischaemic Optic Neuropathy (NAION): A misnomer. Rearranging pieces puzzle to reveal a Nonischaemic Papillopathy caused by Vitreous separation. Ophthalmology. 2015;122:439-442.
14. Hayreh SS. Letter to editor re Parsa & Hoyt Ref 12 above. Ophthalmology. 2015;122: e75.
15. Cullen JF. Letter to editor re Parsa & Hoyt ref 12 above. Ophthalmology. 2015;122:e76.
16. Cogan DG. Neurology of the visual system. Springfield IL. Thomas: 1966; 186 Figure 116.

