Intravitreal bevacizumab for neovascular age-related macular degeneration
Junn R. Pajarillo, MD, Harvey S. Uy, MD, Milagros H. Arroyo MD, MPH
NEOVASCULAR age-related macular degeneration (ARMD) is a leading cause of central-vision loss in people
older than 55 years with an overall prevalence of 14.4% to 36.8%.1-2 The neovascular form accounts for only 10 to 15% of ARMD cases,3-4 but 80 to 90% of all cases of severe visual loss in patients diagnosed with ARMD.4
The natural course of the disease is that of a gradual progressive deterioration and irreversible loss of visual acuity, as well as contrast sensitivity,5which was found to have a significant detrimental impact on the quality of life of these patients.6-7 It is also considered a major public-health issue as the number of new cases is expected to dramatically increase in the next few years.8 High recurrence rates5 and minimal visual gain have made ARMD difficult to manage with conventional laser treatment or photodynamic therapy (PDT) with verteporfin (Visudyne, Novartis Pharmaceutical Corporation, East Hanover, NJ, USA). The discovery and subsequent use of antiangiogenic agents, which specifically block extracellular vascular-endothelial-growth factors (VEGFs),9 ushered in a new era in the treatment of neovascular ARMD. Pegaptanib sodium (Macugen, OSIP, Melville, NY, USA) and ranibizumab (Lucentis, Genentech, South San Francisco, CA, USA) are anti-VEGF drugs for the treatment of neovascular ARMD both approved by the United States Food and Drug Administration (FDA). Large-scale randomized controlled clinical trials have shown that multiple intravitreal injections of Macugen10 or Lucentis11-12 given to patients with neovascular ARMD, regardless of lesion subtype and size, significantly preserved or even improved visual acuity, and were found to be well tolerated with minimal major adverse events.
However, their use has been limited due to high cost. Hence, the need for investigating cheaper alternative antiVEGF compounds that could demonstrate comparable efficacy and safety. Bevacizumab (Avastin, Genentech Inc., CA, USA) is a full-length recombinant humanized monoclonal IgG1 antibody that binds extracellular VEGF and prevents interaction with its receptors on the surface of endothelial cells. It has been approved by the FDA only as first-line combination therapy for metastatic colorectal cancer. However, several off-label, retrospective studies have demonstrated improved clinical outcomes and acceptable
ocular and systemic safety profiles in patients with neovascular ARMD receiving multiple intravitreal
injections of bevacizumab as monotherapy,13-20 or in combination with other treatment modalities.21-22 Patients in these studies showed significant improvement in visual acuity and a decrease in retinal thickness and amount of leakage by fluorescein angiography (FA). More importantly, the procedure was well tolerated with no major complications noted. However, randomized controlled clinical trials are warranted to thoroughly
evaluate its potential effect and safety.This study determined the short-term biologic efficacy and safety of multiple intravitreal injections of bevacizumab in patients with active neovascular ARMD.
METHODOLOGY
A prospective, interventional, placebo-controlled, randomized trial was done involving patients with active
neovascular ARMD consulting at the Medical Retina Clinic of the University of the Philippines–Philippine General Hospital (UP–PGH). Included were patients more than 50 years old diagnosed with subfoveal neovascular ARMD regardless of lesion subtype and with signs of disease activity or progression (hemorrhage, leakage, edema, pigment epithelial detachment) within the past 3 months as evidenced by FA, optical coherence tomography (OCT), and clinical examination. Excluded were patients:
• who had significant media opacities and concomitant retinal/ocular diseases;
• who have had laser treatment (thermal photocoagulation, PDT, transpupillary thermotherapy) or intraocular
surgery within 6 months prior to enrollment;
• who had previous or concomitant therapy with other drugs (antiangiogenic drugs or corticosteroids);
• in whom bevacizumab or any of its components or
fluorescein was contraindicated; and
• who were scheduled for elective surgery within several weeks.
All subjects, and at least 1 relative each, were thoroughly briefed about the study protocol including the risks and benefits, and were asked to sign a comprehensive informed-consent form prior to entry.
The study was approved by the technical and ethical committee of the Research and Implementation Development Office (RIDO) of the UP–PGH. Demographic data were taken and a complete ocular
examination with baseline fundus photo, FA, and OCT was performed. Total lesion size in disc areas was measured using a Topcon licensed software. Patients were randomized using a table of random numbers. They received either 3 consecutive monthly intravitreal injections of 1.25-mg bevacizumab through the pars plana or sham injections. All bevacizumab vials were stored at the recommended temperature and newly opened prior to injection. The vials were discarded immediately after the procedure. Topical moxifloxacin hydrochloride (Vigamox, Alcon Laboratories Inc., Forth Worth, TX, USA) given at 1 drop every 15 minutes starting 1 hour prior to the procedure was used as preoperative antibiotic prophylaxis. Sterile drapes were placed and all eyes were prepared in a standard manner using 5% povidone iodine applied as lid scrub. Bladed speculums were
used to retract the lids and lashes. The pars plana was entered approximately 3.5 to 4 mm from the superior corneal limbus using a gauge-30 needle directed toward the middle of the
globe. Upon withdrawal of the needle, a sterile cotton pledget was used to apply pressure on the
puncture site for hemostasis, and topical moxifloxacin was again placed on the eye. Patients were then instructed to administer moxifloxacin eye drops at 1 drop 4 times a day immediately after each injection for
a total of 7 days. Follow-ups were made on the second, fourth, eighth, and 12th weeks postinjection. Sham
injections consisted of all the steps of the procedure except for actual needle penetration.
Best-corrected visual acuity (BCVA) using standard ETDRS chart: letter-by-letter counting, central 1 mm
of macular thickness as measured by OCT (Stratus OCT, Carl Zeiss Meditec, Dublin, CA, USA), including a complete ophthalmologic examination, were recorded at each visit. Ocular/Periocular or systemic drugrelated side effects or toxicities and iatrogenic complications were noted. All examinations were done by masked outcome assessors.
STATISTICAL ANALYSIS
All numerical continuous data were summarized using descriptive statistics (percentage, frequency
distribution, and measures of central tendency). T-test was used to compare continuous numerical variables of the 2 groups while discrete categorical variables were compared using chisquare or Fisher’s exact tests.
To test for changes in continuous and numeric independent variables, repeated measures analysis of variance (ANOVA) was employed. Intentionto-treat analysis was done using lastobservation-carried-forward (LOCF) method to impute for missing data. To determine the degree of association between two continuous
numerical variables, Pearson Product Moment Correlation was computed. The computed r-value was compared against the criteria for degree of association.



All statistics were carried out using the licensed statistical software, Statistical Package for the Social Sciences (SPSS Version 15). Hypothesis testing was carried out at a 0.05 level of significance.
RESULTS
A total of 30 eyes were included in the study. Three patients were unable to complete the prescribed number
of follow-ups, one in the bevacizumab group because of acute inflammatory reaction after the second injection, and two in the control group for reasons not related to the treatment. The outcomes from all 30 eyes were included in the final analysis. The patients had a mean age of 68.33 ± 8.37 years, baseline visual
acuity of 24.33 ± 13.39 letters, central macular thickness of 389.80 ± 156.64 µ, and lesion size of 15.27 ± 8.78 disc areas (Table 1). None of the patients had previous photodynamic therapy using verteporfin (Visudyne) or any intravitreal anti-VEGF or steroid treatment at the time of enrollment. There was no statistically significant difference between groups in terms of age, sex distribution, duration of symptoms, and frequency of previous treatments for neovascular ARMD. Differences between baseline visual acuity, central macular thickness, lesion size, and intraocular pressure for all eyes examined were also not statistically significant. Visual acuity
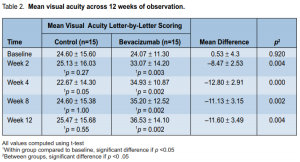
There was a significant increase in mean visual acuity (VA) from baseline for the treatment group across alltime periods (p = 0.002) (Table 2). The slope of the line showed that the increase was most marked during the first 2 weeks of observation and reached a plateau thereafter (Figure 1). There was no significant change in the mean visual acuity from baseline for the control group across all time periods (p = 0.55). The visual-acuity scores were

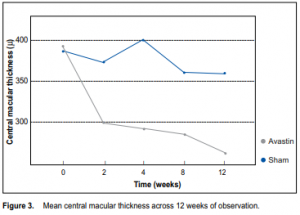
significantly higher for the treatment group than for the control group across all time periods (p < 0.01) (Table 2). The mean difference between the 2 groups at the end of the study was 11.60 ± 3.49 letters (p = 0.004). There was no significant difference between the number of patients with visual loss ≤ 15 letters between the 2 groups at the end of the study (p = 0.12) (Table 3, Figure 2). There was, however, a significantly larger proportion of patients in the treatment group who had visual gain ≥ 5 (p = 0.003) and ≥ 10 letters (p < 0.001). Central macular thickness There was a significant decrease in mean central macular thickness from baseline for the treatment group across all time periods (p < 0.001) (Table 4). The slope of the line (Figure 3) showed that the decrease was most marked during the first 2 weeks of observation and remained the same thereafter. There was no significant change in the central macular thickness from baseline for the control group across all time periods (p = 0.07). The mean central macular thickness was significantly reduced in the treatment group compared with the control group across all time periods. The mean difference between groups at the end of the study was 95.93 ± 77.86 µ (p = 0.004) (Table 4). Patients with better baseline visual acuity and less macular
thickness had significantly better final visual acuity at the end of 12 weeks of observation (r = +0.72, p < 0.001 and r = –0.39, p = 0.03 respectively). Eyes with smaller lesion and patients with shorter duration of symptoms at baseline had better final visual acuity at the end of the study, but the correlation was not statistically significant (Table 5). There were no major ocular or systemic drug-related side effects or iatrogenic complications for both groups except for a single case of a severe inflammatory response a few hours after the second injection of bevacizumab. A total of 44 intravitreal bevacizumab injections and 41 sham injections were administered. The overall rate of adverse events for the control group was 0% (0 of 41) compared with 2.27% (1 of 44) for the treatment group (Table 6).
DISCUSSION
Clinical evidence has implicated VEGF-A in the pathogenesis of neovascular ARMD. VEGFs are potent mitogens for vascular endothelial cells9 naturally present in the retinal pigment epithelium and needed for a variety
of physiologic responses. Overexpression of VEGFs results in new-vessel formation and increased vascular permeability, both hallmarks of neovascular ARMD. Bevacizumab is a full-length antibody derived from the
same murine antibody as that of ranibizumab. Bevacizumab blocks all isoforms of extracellular VEGF-A and prevents interaction with its receptors on the surface of endothelial cells; therefore, avoiding its undesirable effects. Its use has been on a compassionate basis. In this study, monthly intravitreal injections of bevacizumab produced a statistically significant and clinically meaningful benefit compared to no treatment in patients with active neovascular ARMD. Visual acuity increased by an average of approximately 10 letters (2
lines) on the ETDRS chart. The benefit was most apparent during the first 2 weeks after initiation of treatment and was sustained until the end of the study period. Patients who received treatment were 5 times more likely to gain at least 5 letters, and 10 times more likely to gain at least 10 letters. Results were similar to those of other reported case series involving the use of bevacizumabin which the average gain of visual acuity after multiple injections was approximately 1 to 2 lines (5 to 10 letters by ETDRS) in 8 to 28 weeks.14-18, 20-21 This was also comparable with the effects observed for those treated with ranibizumab.11-12 These provided evidence of anti-VEGF efficacy in stabilizing and improving vision. Central macular thickness in the treatment group was reduced by an average of 100 µ at the end of the study and paralleled the increase in visual acuity observed. A marked reduction in central macular thickness may explain the visual outcomes observed in this study. Similar outcomes have been demonstrated by other studies wherein the average range of decrease in central macular thickness was 41 to 127 µ in 12 to 24 weeks after multiple doses of intravitreal bevacizumab.14-21 This study provided indirect biologic evidence of the antipermeability property of the drug. While its antiangiogenic effect was not well demonstrated, actual CNV sizes at baseline and during subsequent follow-up examinations were not documented. Stabilization, particularly regression, of the neovascular process is desired so as to achieve disease remission. Longer periods of observation and detailed clinical, angiographic, and tomographic studies are needed to assess this effect. This study also evaluated the effects of several baseline characteristics with final visual acuity. Patients with better preoperative visual acuity also had better vision at the end of the study than those with poorer visual acuity at baseline. However, the amount of change from baseline was not dependent on the initial level of visual acuity. Baseline lesion size and duration of symptoms also had no significant effect on the final visual acuity. These data showed that a broad
range of patients could benefit from this treatment. Bevacizumab is a full-length recombinant humanized
monoclonal IgG1 antibody used primarily as a chemotherapeutic agent for metastatic colorectal carcinoma and is administered via systemic intravenous injections. The preparation is unpreserved and contains no ingredients that are known to be toxic to the eye.14
However, it was not formulated as an intraocular agent and did not undergo rigid safety trials, hence, its ocular
safety is not well established. There are only a few case reports, both in animals and humans, that evaluated the drug’s or any of its components’ direct effects on ocular tissues. Electrophysiologic and clinical studies showed that bevacizumab appears to have no observable toxic effects on adjacent ocular tissues.20, 23-24 The International Intravitreal Bevazicumab Safety Survey conducted in 2006 reported overall rates of ocular and systemic drug-related and procedure-related adverse events of less than 0.21%.25 Published reports of several case series have also shown low rates of major adverse events resulting from the drug’s direct effects or from the procedure.14-20 The rate of complications for other types of anti-VEGF drugs was less than 2%.1 The overall rate of adverse events in this study was 2.67% for the treatment group, slightly higher compared with the rate in other studies but still substantially low. There were no cases of undesired procedure-related events such as infectious endophthalmitis, vitreous hemorrhage, traumatic lens injury, or retinal detachment. Still, it is critical that all treating ophthalmologists carefully adhere to an appropriate aseptic technique, educate patients well, and closely monitor them after each injection. Noteworthy was the single case of a severe inflammatory response a few hours after the second cycle of bevacizumab. This was manifested as conjunctival chemosis, intense anterior-chamber inflammation with grade 1 hypopyon, mild vitreous cellularity, and decreased visual acuity. Microbiologic studies of anterior-chamber and vitreous specimens were negative for any organism, and prompt resolution of symptoms occurred after 4 days of topicalsteroid treatment. Several cases have been reported that showed an increased inflammatory response after bevacizumab25 or ranibizumab26 injection. It is currently unknown what component of the drugs causes this reaction or if there are any identifiable predisposing factors. It has been previously hypothesized that the uveitis associated with
bevacizumab and ranibizumab is probably a result of a protein, which is not completely humanized, being exposed to the immune system and inciting a reaction.27 In summary, this study supported the growing body of evidence that intravitreal injections of bevacizumab can result in short-term anatomical and functional
improvement with minimal adverse events for patients with neovascular ARMD. However, long-term studies with more subjects are needed to thoroughly evaluate the efficacy and safety of the drug and compare visual acuity between treated and untreated eyes over the long term. The end points for treatment, efficacy of combined modalities, as well as the cumulative effects of multiple injections including the compounded risk for iatrogenic complications are not known at this time.
References
1. Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment in the United States. Arch Ophthalmol 2004; 122: 477-485.
2. Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1992; 99: 933–943.

