Proposed New Retinopathy of Prematurity Screening Criteria: Evidence for Including Older and Heavier Filipino Premature Babies
Kristine Corpus, MD, Jose Melvin Jimenez IV, MD, Rachelle Anzures, MD, Rena Ivy Bascuna, MD, Ricardo Ventura, MD, Macario Reandelar Jr., MD, MSPH, Retinopathy of Prematurity Working Group
INTRODUCTION
Retinopathy of prematurity (ROP) is a retinal disease of preterm infants with a wide spectrum of presentation, from mild transient retinal changes to severe vaso-proliferation, which may lead to retinal detachment.1,2 It is a significant and a potentially avoidable cause of childhood blindness.3 Sixty percent of childhood blindness in developing countries, the Philippines included, is due to ROP.4,5 We are in- cluded among the nations who are at high risk for blindness due to ROP, with 9-60 blind infants per 1,000 births due to inadequate neonatal care and ROP screening.6 Prompt screening and detection has been established to reduce complications of ROP among infants at risk.7 The financial cost of supporting one child blinded by ROP has been estimated to exceed the cost of screening and treatment; hence, the need for screening appropriate for the population of babies in a given locale.8,9 However, ROP screening is only effective if no baby with severe ROP is missed.10
Published studies showed “younger and smaller” babies with ROP were observed in developed countries whereas “older and bigger” babies with ROP were reported in developing nations.5-21 In 2005, Gilbert reported that 13% of 1,091 infants with severe ROP from 10 developing countries were missed because these exceeded the screening cut- off of ≤32 weeks gestational age (GA) and ≤1,500 grams birth weight (BW).5 In 2006, Chen reported a missed-out rate of 16.2% out of the 114 Chinese babies seen with severe ROP and who were also outside the same 32-week and 1,500-gram cut-off.12 A significant number of published studies from various developing countries have described these babies with >32 weeks GA and >1,500 grams BW, with the oldest at 36 weeks GA and heaviest at 2,600 grams BW and with missed-out rates of 6.6-28.3%.5-21 As reported in the 3rd World ROP Congress in Shanghai in 2012,many developing countries, such as China, India, Thailand, and Vietnam, have already included older and heavier preterm babies, as old as 33-36 weeks GA and 1,750-2,000 grams BW, in their national ROP screening criteria.5,12-14, 20, 25-28 These older and bigger babies fell outside our existing Philippine Academy of Ophthalmology-Philippine Pediatric Society local screening criteria of GA ≤32 weeks and BW ≤1,500 grams.11 These data were also supported by anecdotal reports from our local retina specialists and pediatric ophthalmologists who have treated babies with severe ROP born >32 weeks GA and >1,500 grams at birth and who were not screened early because they were above our existing screening criteria.
Although local studies on ROP have validated the applicability of the local ROP screening criteria, there is no evidence at present that have determined whether babies with ROP >32 weeks GA and >1,500 grams BW are missed.17-19 We need to establish our missed-out rates as our institutional ROP incidence rates do not truly reflect our current ROP scenario.
This study determined if preterm babies >32 weeks GA and >1,500 grams BW developed ROP and who were thus “missed” with the existing local criteria. It also determined the incidence of these “missed babies” and described their profile (GA, BW, severity of ROP, and associated risk factors). The data provided information on whether there is a need to modify the 2005 local ROP screening criteria and determined the appropriate upper limit for GA and BW for screening that can provide a safe and efficient “cut-off” for detecting severe ROP.
METHODOLOGY
This is a multi-center, retrospective cohort, observational study using data on ROP screening from the medical records of infants in the Neonatal Intensive Care Unit (NICU), nursery, ward, and outpatient department (OPD) of 4 institutions for the year 2011-2013. The collaborating institutions were St. Luke’s Medical Center Quezon City (SLMC), Dr. Jose Fabella Memorial Hospital (Fabella), East Avenue Medical Center (EAMC), and Ospital ng Makati (OSMAK). In these institutions, the respective Department of Ophthalmology has been proactively encouraging their Department of Pediatrics to refer preterm infants with ≤36 weeks GA for ROP screening. The screening criteria were deliberately set past the 32-week cut-off to avoid missing babies.
Institutional review board approval was not required because the data collected pertained to routine clinical care of preterm infants (e.g. GA, BW) and ophthalmologic care (e.g. ROP diagnosis, stage, and treatment) of babies referred for ROP screening. These data were the results obtained from previous ROP screening exams and treatments that adhered to standardized protocols and the prerequisite of which entailed parental consent.
Data on ROP from the medical records of previously screened babies, ≤36 weeks GA, regardless of BW and status of clinical course, from private and charity services were included. Data collated included the following: GA by last menstrual period, BW, risk factors (maternal and neonatal), and results of the ROP screening. The worst ROP diagnosis per eye and per infant was recorded. Outcome measures in- cluded: (1) incidence (missed-out rate) of preterm babies >32 weeks GA and >1,500 grams BW with ROP; (2) profile of the “missed babies” with ROP (GA, BW, severity of ROP, and risk factors (maternal and neonatal); and (3) proposed screening criteria
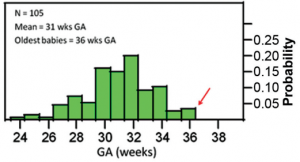
Figure 1. Distribution of babies with ROP according to gestational age (GA).
that included babies with severe ROP. In this study, Type 1 ROP and Stages 4-5 ROP were referred to as “severe ROP.”
Based on the assumption that the incidence of our missed babies with ROP will more or less mirror that of the larger retrospective study of Gilbert5 with an incidence of 13%, the sample size was calculated at 483 with 95% reliability and a maximum allowable error of 3%. Descriptive statistics were utilized and categorical variables analyzed using Chi-square test with the level of significance set at p≤0.05 and calculated using SPSS software for Windows (SPSS Inc, Chicago, Illinois, USA).
RESULTS
Patient Characteristics
Data from 762 preterm babies, ≤36 weeks GA, were included from the 4 institutions. Of the 762 babies, 105 or 13.8% had ROP in any stage. Among the 105 babies, 13 infants (12%) had severe ROP. Among the infants with severe ROP, 10 (77%) had type 1 ROP and 3 (23%) had stage 4. There was no stage 5.
Of the 105 babies with ROP, the mean GA and BW were 31 weeks and 1,373 grams, respectively. There were babies with ROP above 32 weeks GA and above 1,500 grams BW, with the oldest at 36 weeks and heaviest at 2,500 grams (Figures 1 and 2). Majority (38.1%) of the 105 babies came from Fabella. There was, however, no statistically significant difference (p=0.73) in the incidence of ROP among the 4 institutions.

Figure 2. Distribution of babies with ROP according to birth weight (BW).
Profile of all babies with ROP
Twenty-eight (27%) of the 105 babies with ROP were 33-36 weeks GA while 32 (30%) had BW >1,500 grams. Although majority of ROP-positive infants were in the younger GA group, older babies >32 weeks GA were also observed (Table 1). Three percent of babies born at 33-34 weeks GA had stage 3 ROP while 0.5% of babies born at 31-32 weeks GA had stage 4 ROP. Likewise, babies with ROP heavier than 1,500 grams at birth were also reported. Stage 3 ROP was seen in 1.4% of babies weighing 2,000- 2,500 grams and in 3.1% weighing 1,600-1,999 grams. Stage 4 ROP was seen only in babies weighing less than 1,500 grams.
The incidence of pre-plus in babies 33-34 weeks GA was 1.5%, similar to those in the younger gestational age. The incidence of plus disease in the same GA was 0.7%, slightly higher than those in the 31-32 weeks GA. Babies weighing 1,600-2,500 grams had pre-plus at 1.5% and plus disease at 1% (Table 2). Although 3 babies (0.4%) with stage 4 ROP were noted to be ≤32 weeks GA and ≤1,500 grams, type 1 ROP was seen in 2 babies (0.7%) born at 33- 34 weeks GA and in 3 babies (1%) weighing 1,600- 1,999 grams at birth. Infants with type 2 ROP were observed to be present even at >32 weeks GA and >1,500 grams BW (10.9% and 9.9% respectively) (Table 3).
The oldest preterm babies that underwent treatment such as intravitreal injection of beva- zicumab (IVB) and laser indirect ophthalmoscopy (LIO) were born at 33-34 weeks GA with an incidence of 0.4% and 1.9% respectively. Heavier babies with BWs of 1,600-1,999 grams had IVB injection (0.7%) and LIO treatment (0.7%) for those weighing 2,000-
Table 1. Distribution of babies according to stage of ROP.
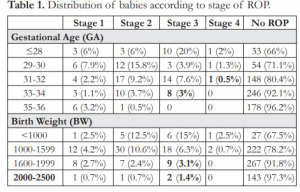
Table 2. Distribution of babies according to pre-plus and plus disease.
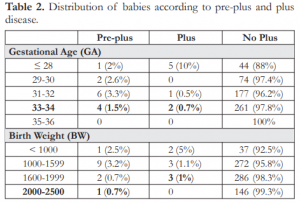
Table 3. Distribution of babies according to severity of ROP.
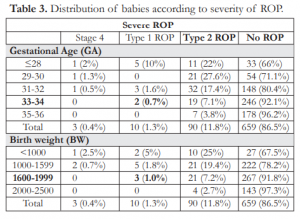
Table 4. Distribution of babies according to treatment of ROP.
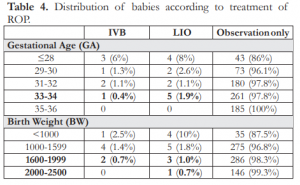
2,500 grams (Table 4). None of the babies underwent surgery. Although the oldest babies with aggressive posterior retinopathy of prematurity (APROP) were born at 31-32 weeks GA (1.1%), there was 1 (0.3%) baby with APROP weighing 1,600-1,999 grams at birth (Table 5).
Table 5. Distribution of babies according to presence of aggressive posterior ROP (APROP).

Table 6. Presence of risk factors among missed babies (33-36 weeks and >1,500 grams).
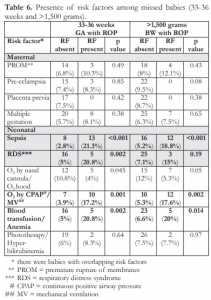
Table 7. Table 7. Distribution of babies and presence of ROP and risk factor according to institution.
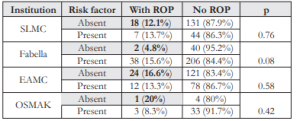
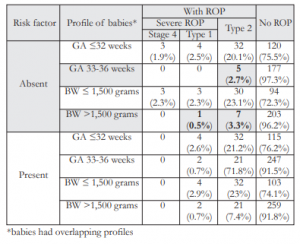
Table 8. Distribution of babies according to presence of risk factor, GA, BW, and type of ROP.
When sepsis was present, the incidence of ROP among babies >32 weeks GA was significantly increased (21.3% vs 2.8%) (Table 6). The same pattern was also seen with other risk factors when present among babies >32 weeks GA compared to absent risk factors such as respiratory distress syndrome (RDS), oxygen (O2) supplementation via continuous positive airway pressure (CPAP) or mechanical ventilation (MV), and blood transfusion and anemia. Likewise, the incidence of ROP among babies >1,500 grams BW was also significantly higher than in the absence of sepsis (18.8% vs 5.2%) or O2 supplementation via CPAP or MV (17.6% vs 5.3%). All comparisons were statistically different.
There were also babies who developed ROP in the absence of risk factors. Of the 105 babies with ROP, 43% had no risk factor identified and 57% had at least one. The incidence of ROP among infants with and without risk factors was not statistically different (p=0.67). Of the 45 babies who developed ROP in the absence of risk factors, 18, 2, 24, and 1 infants were from SLMC, Fabella, EAMC, and OSMAK respectively. The incidences of ROP among the different groups, with and without risk factors, were not statistically different (Table 7). Among the 45 babies with ROP but with no associated risk factor, 5 infants (2.7%) had type 2 ROP and were born at 33- 36 weeks. Likewise, 7 infants (3.3%) had type 2 ROP and 1 baby had type 1 ROP and all were >1,500 grams at birth (Table 8).

Table 9. Profile of the 17 missed babies.
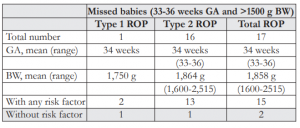
Missed Babies with ROP
When the cut-off of ≤32 weeks GA and ≤1,500 grams BW was applied to the 105 babies with ROP, there were 16.2% (17 infants) with GA of 33-36 weeks and BW >1,500 grams. These babies were missed with the existing screening criteria (Figure 3).
Of the 17 missed babies, the mean GA was 34 weeks and mean BW 1,858 grams, with the oldest and heaviest at 36 weeks and 2,515 grams respectively (Table 9). Although majority (16 babies) had type 2 ROP and most (15 babies) had risk factors, there were 2 babies with no risk factor. The first baby weighing 1,875 grams was born 33 weeks and had type 2 ROP. The second infant weighing 2,200 grams was born 34 weeks and had type 1 ROP and underwent LIO.
DISCUSSION
Among the 105 infants with ROP, there were indeed older and heavier babies with >32 weeks GA and >1,500 grams BW. The oldest was at 36 weeks and the heaviest at 2,500 grams. The average GA and BW, as well as the oldest and heaviest babies reported in this study, did not differ largely from those reported in the local ROP studies,22-24 nor from published studies of other Asian countries.5-21
The oldest babies who had type 1 ROP (0.7%) were born at 33-34 weeks GA. They received either LIO or IVB treatment (2.3%). Of these, 3% had stage 3 ROP and 2.2% had pre-plus/plus disease. The heaviest babies weighing 1,600-1,999 grams had type 1 ROP (1%) and APROP (0.3%). They also underwent either LIO or IVB (1.7%).Of these, 4.5% had stage 3 ROP and 1.7% had pre-plus/plus disease. The results showed that the oldest and heaviest babies with severe ROP were almost consistently and significantly reported at <35 weeks GA and <2,000 grams BW.
The risk factors that were significantly associated with higher incidences of ROP among 33-36 weeks GA and >1,500 grams BW included sepsis, RDS, O2 supplementation by CPAP or MV, and blood transfusion. However, there were babies (43%) who developed ROP with no associated risk factors. Among these babies were infants with GA >32 weeks and BW >1,500 grams.
When the existing local screening cut-off of ≤32 weeks GA and ≤1,500 grams BW was applied to our population, 17 babies (16.2%) were missed. This does not fall short from the 13% missed-out rate of Gilbert5 involving 10 developing countries and the 16% missed- out rate of Chen’s study12 on Chinese babies or from other missed-out rates from other low-income Asian countries.5-21 Of the 17 missed babies, there were 2 infants who had no risk factor, one of which had type 1 ROP and needed treatment. Therefore, these 2 babies would still be missed even if risk factors were considered. Although there were only 2 babies with ROP that were missed, they still merit screening as the consequences of blindness is devastating. According to the American Academy of Pediatrics, “the goal of an effective ROP screening program is to identify ALL infants who need treatment.”1
It appears that to “catch” the 17 missed babies in this study, the screening criteria should include the oldest and heaviest babies with severe ROP, approximately <35 weeks GA and <2,000 grams BW. After applying these proposed criteria to our population, we obtained a missed-out rate of 2% from the previously measured 16.2% (Figure 4). There were still 2 babies missed, both with type 2 ROP; however, these infants could still be screened with the inclusion
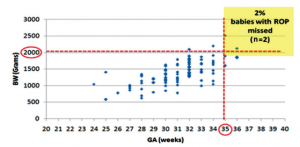
Figure 4. Distribution of babies with ROP and the cut-off <35 weeks GA and ≤2,000 grams BW (proposed screening criteria).
of risk factors as both had history of sepsis, blood transfusion, and O2 supplementation.
In conclusion, the existing ROP screening criteria of ≤32 weeks GA and ≤1,500 grams BW missed babies with severe ROP, including those without risk factors; and, thus, need to be modified. Based on the above findings, we recommend ROP screening for premature Filipino babies: (1) <35 weeks GA and/or <2,000 grams BW, and (2) babies ≥35 weeks GA or ≥2,000 grams BW with the presence of risk factors.
The incidence of pre-plus in babies 33-34 weeks GA was 1.5%, similar to those in the younger gestational age. The incidence of plus disease in the same GA was 0.7%, slightly higher than those in the 31-32 weeks GA. Babies weighing 1,600-2,500 grams had pre-plus at 1.5% and plus disease at 1% (Table 2). Although 3 babies (0.4%) with stage 4 ROP were noted to be ≤32 weeks GA and ≤1,500 grams, type 1 ROP was seen in 2 babies (0.7%) born at 33- 34 weeks GA and in 3 babies (1%) weighing 1,600- 1,999 grams at birth. Infants with type 2 ROP were observed to be present even at >32 weeks GA and >1,500 grams BW (10.9% and 9.9% respectively) (Table 3).
As this is a retrospective study, several limitations are inherent. We did not attempt to address the specific details of the maternal and neonatal risk factors affecting ROP, issues of variation in survival rates, levels of neonatal care and ophthalmologic expertise, as well as the economic implications of increasing the number of babies requiring ROP screening. The new recommendations aim to widen the current screening criteria, using the two consistent and established risk factors for ROP – prematurity and low birth weight.29 As ROP screening programs are evolving and may have intrinsic defects, such as over referral and under referral,10 we recommend reevaluation of the proposed criteria, if implemented, every 1-2 years, utilizing data from a prospective study.
MEMBERS OF THE RETINOPATHY OF PREMATURITY WORKING GROUP (ROPWG)
Chair: Pearl Tamesis-Villalon
Members: Rachelle Anzures, Jubaida Aquino, Milagros Arroyo, Ma. Cecilia Arenal, Joanne Bolinao, Johanna Cervantes, Carlos Chua, Kristine Corpus, Fay Charmaine Cruz, Jose Melvin Jimenez IV, Marie Joan Loy, Emilio Macias, Andrea Kristina Pajarillo, Junn Pajarillo, Maria Victoria Rondaris, Alvina Pauline Santiago, Darby Santiago, Rena Ivy Bascuna, Ricardo Ventura.
ACKNOWLEDGMENT
The authors would like to extend their deepest gratitude to Drs. Mary Christine Alice Tumale, head of the neonatology section; Darlene de Leon Salloman, neonatology fellow of Jose Fabella Memorial Hospital; Marian Ana and Angela Zuno, ophthalmology residents of Ospital ng Makati for their invaluable assistance in the data collection.
REFERENCES
1. American Academy of Pediatrics, American Academy of Ophthalmology, American Association of Pediatric Ophthalmology and Strabismus, and American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2013;131:189.
2. Chaudhari S, Patwardhan V, Vaidya U, et al. Retinopathy of prematurity in a tertiary care center – incidence, risk factors and outcome. Indian Pediatr 2009;46:219-224.
3. Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk, and implications for control. Early Human Development 2008;84:77-82.
4. Gilbert C and Foster A. Childhood blindness in the context of Vision 2020: the right to sight. Bull World Health Organ 2001;79:227-232.
5. Gilbert C, Fielder A, Gordillo L, et al, International NO-ROP Group. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics 2005;155:e518-e52.
6.World Health Organization, March of Dimes, The Partnership for Maternal, Newborn, and Child Health and Save the Children. Born too soon: the global action report on preterm birth. 2012;1-124.
7. Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity. Arch Ophthalmol 2003;121:1684-1696.
8. Azad R. Retinopathy of prematurity: a giant in the developing world. Indian Pediatr 2009;46:211-2.
9. Elder JE. Is it time to review the screening guidelines for retinopathy of prematurity? J Paediatr Child Health 2008; 44:159-160.
10. American Academy of Pediatrics, American Academy of Ophthalmology, and American Association of Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2013;131:189-195.
11. Philippine Pediatric Society, Philippine Academy of Ophthalmology, Vitreo-Retinal Society of the Philippines, and Philippine Society of Pediatric Ophthalmology and Strabismus. A joint statement on screening guidelines on retinopathy of prematurity. Policy Statements of the Philippine Pediatric Society, Inc. Series 2005,1(1-8):12-14.
12. Chen Y, Li X. Characteristics of severe retinopathy of prematurity patients in China: a repeat of the first epidemic? Br J Ophthalmol 2006;90:268-271.
13. Trinavarat A, Atchangeeyasakul L, Udompunturak S. Applicability of American & British criteria for screening of retinopathy. Jpn J Ophthalmol 2004;48:50-53.
14. Rasa S. Retinopathy of prematurity: is it time to change screening limits in Lithuania? Vilnius University Children’s Hospital, World ROP Congress, 2006.
15. Jalali S, Matalia J, Hussain A, Anand R. Modification of screening criteria for retinopathy of prematurity in India and other middle-income countries. Am J Ophthalmol 2006;141:966-968.
16. Vinekar A, Dogra M, Sangtam T, et al. Retinopathy of prematurity in Asian Indian babies weighing greater than 1250 grams at birth: ten-year data from a tertiary care center in a developing country. Indian J Ophthalmol 2007;55:331- 336.
17. Sanghi G, Dogra MR, Pranab D, et al. Aggressive posterior retinopathy of prematurity in Asian Indian babies: spectrum of disease & outcome after laser treatment. Retina 2009; 29:1335-1339.
18.Shah PK, Narendran V, Kalpana N, Gilbert C. Severe retinopathy of prematurity in big babies in India: history repeating itself? Indian J Pediatr 2009;76:801-804.
19. Erdeve O, Atasay B, Arsan S, et al. Restricted universal guidelines for ROP screening: a possible misguidance for middle-income countries. Turk Med Sci 2010;40:791-796.
20. Zin AA, Moreira MEL, Bunce C, et al. Retinopathy of prematurity in 7 neonatal units in Rio de Janeiro: screening criteria and workload implications. Pediatrics 2010;126:e410- e417.
21. Ghairabeh A, Khassawneh M, Khriesat W, et al. Adopting western retinopathy of prematurity screening programs in eastern countries, are we screening properly? Middle East Afr J Ophthalmol 2011;18:209-213.
22. Arroyo MH, Camonias DL, Monzon-Pajarillo AK, et al. Criteria for the timing of the initial retinal examination to screen for retinopathy of prematurity. Philipp J Ophthalmol 2010;35:15-19.
23. CerdanaHG,LiaoCS,MaciasIIIEL,NanagasMLR.Results of initial screening for retinopathy of prematurity at a tertiary hospital. Philipp J Ophthalmol 2010;35:56-60.
24. Ladores CS, Banzon M. Status of screening for retinopathy of prematurity in a tertiary hospital. Philipp J Ophthalmol 2010;35:61-64.
25. National Neonatology Forum India. Evidence-Based Clinical Practice Guidelines. October 2010
26. Gilbert C. ROP: International perspectives. Shanghai, China 2012; 3rd World ROP Congress.
27. Nguyen XT. Epidemiology of ROP in Vietnam. Shanghai, China 2012; 3rd World ROP Congress.
28. Narendram V. ROP screening guidelines: Indian perspective. Shanghai, China 2012; 3rd World ROP Congress.
29. Stout A and Stout T. Retinopathy of prematurity. Pediatr Clin N Am 2003;50:77-87.

