Vector Analysis Outcomes after Femtosecond Laser In-Situ Keratomileusis (FS-LASIK) Versus Small Incision Lenticule Extraction (SMILE) for Moderate Myopic Astigmatism
John Arvin B. delos Reyes, MD, Kathrina Therese O. Mendoza, MD, Reginald Robert G. Tan, MD
Tan Eng Gee Eye Institute, St. Luke’s Medical Center – Global City, Taguig, Philippines
Correspondence: John Arvin B. delos Reyes, MD
Clinic Address: Borough LASIK Center, 2F North Parking Building, SM Mall of Asia Wellness Zone, Pasay City, Philippines
Clinic Phone Number: +639177072306
E-mail Address: jarvindlr@gmail.com
Disclosure: The authors report no conflict of interest.
Error of refraction, particularly myopia, is the leading cause of visual impairment worldwide. The global prevalence of 22.9% for myopia and 2.7% for high myopia in 2000 is predicted to increase to 49.8% and 9.8%, respectively, by 2050.1 Treatment has evolved from spectacles and contact lenses to refractive surgical procedures. Over the years, different types of laser refractive surgeries have been developed to improve visual outcomes and reduce post-operative complications.
Currently, the most performed refractive surgical procedure is laser-assisted in-situ keratomileusis (LASIK).2 This procedure involves the creation of a corneal flap and the use of an excimer laser to ablate the stromal bed to change the cornea’s refractive power. The femtosecond laser is one of the most revolutionary inventions in recent medical technology and has been used mainly in the field of ophthalmology for the creation of a precise and reproducible LASIK flap. However, even with LASIK’s high success rate, occasional concerns such as dry eye, induction of higher-order aberrations causing glares and halos, and flap-related issues remain.3
Small incision lenticule extraction (SMILE) has gained widespread acceptance because it is flapless, has less impact on the ocular surface and corneal innervation, and has the potential advantage of leaving the cornea more biomechanically stable as compared to LASIK.4 Other advantages are the use of a smaller incision, the avoidance of flap-related complications, and the induction of fewer higher- order aberrations.3,5 This procedure was approved by the US Food and Drug Administration (FDA) in 2018 for treating -1.00 to -8.00 diopters (D) of myopia and up to 3.00 D of astigmatism. Since then, it has demonstrated promising visual and refractive outcomes with an excellent safety profile comparable to femtosecond-assisted LASIK.3,4
The introduction of eye trackers into modern excimer laser platforms has improved LASIK results significantly. The MEL 80 excimer laser (Carl Zeiss Meditec, Germany) utilizes an iris registration camera that tracks the eye and maintains centration throughout ablation. However, this centration method is not utilized in SMILE, and therefore treatment alignment is dependent on the skill of the surgeon.6 However, centration with SMILE has not been established to be inferior than excimer-based treatments like LASIK as long as a depurate procedure is applied and attention to treatment centration is given after placement of suction and before treatment commences.7,8
Non-randomized comparative studies of the visual, refractive, contrast sensitivity, and aberrometric outcomes of LASIK and SMILE have been performed. Most of the studies found no significant differences in visual and refractive outcomes between the two techniques.3,5-7,9-11 However, surgeon-dependent centration and the lack of cyclotorsion control in SMILE have raised concern on its ability to adequately correct moderate to high levels of myopic astigmatism.7 A previous study on vector analysis of astigmatic correction between SMILE and LASIK in patients with low to moderate astigmatism showed similar residual spherical equivalent in both groups but significantly higher residual cylinder after SMILE.3 In addition, mean residual astigmatism was lower in the low- cylinder subgroup than in the high-cylinder subgroup.5
Predicting the outcome of astigmatism is more complex because astigmatism involves two aspects: power and axis. Astigmatism can, therefore, be treated as a vector because it has a magnitude and direction. There are three fundamental vectors that determine the effectiveness of astigmatism correction following laser refractive surgery: target- induced astigmatism (TIA) vector, surgically- induced astigmatism (SIA) vector, and difference vector (DV). Applying the vector method, astigmatism can be decomposed into orthogonal vectors, and it can indicate refractive outcomes using simple mathematical formulas. This study aimed to compare astigmatic correction among eyes that underwent femtosecond-LASIK or SMILE for moderate myopic astigmatism.
METHODS
This single-center, comparative, retrospective, cohort study was approved by the Institutional Scientific Review Committee and the Institutional Ethics Review Committee. The target population were all adult myopic patients who underwent femtosecond-LASIK or SMILE for the first time from January 2014 to January 2020 with preoperative corrected distance visual acuity (CDVA) of 20/20 or better, manifest cylinder refraction of -0.75 to -3.0 D, and at least 3 months of postoperative follow-up at a tertiary medical center. Patients with a history of ocular surgery, those who developed any intraoperative and postoperative complication after femtosecond- LASIK or SMILE, or those who had topography or wavefront-guided treatments were excluded from the study. The potential study patients were identified from a hospital database of patients who underwent femtosecond-LASIK or SMILE. All patients who satisfied the screening criteria were included. Medical records of eligible patients who underwent femtosecond-LASIK or SMILE were reviewed. The following data were collected: age, gender, baseline preoperative refraction, postoperative visual acuity, and postoperative refraction of at least 3 months from treatment.
Manifest and cycloplegic refractions were done by 1 of 2 in-house optometrists. Diagnostic eye exams included ATLAS corneal topography (Carl Zeiss Meditec, Germany), Pentacam Scheimpflug imaging (OCULUS, Germany), IOL Master 700 biometry (Carl Zeiss Meditec, Germany), WASCA aberrometry (Carl Zeiss Meditec, Germany) and specular microscopy (Konan, Japan).
All study patients underwent routine bilateral simultaneous femtosecond-LASIK (MEL 80 and VisuMax 500, Carl Zeiss Meditec, Germany) or SMILE (VisuMax 500, Carl Zeiss Meditec, Germany) done by 1 of 6 in-house refractive surgeons. No nomograms were used for all patients during treatment.
Primary outcome measures included analysis of the vector parameters and astigmatic graphs based on refraction at 3 months after femtosecond-LASIK or SMILE. Secondary outcome measures included visual and refractive outcomes such as postoperative visual acuity, refraction, treatment safety, and treatment efficacy at least 3 months after the procedures.
Vector Analysis
Vector analysis of astigmatic correction was done using the Alpins Statistical System for Ophthalmic Refractive Surgery Techniques (ASSORT®) VectrAKTM Astigmatic Vector Calculator.12,13 All refractive values were converted to positive cylinder form.
The following vector parameters, originally defined and described by Alpins, were used in the study12:
- Target-induced astigmatism (TIA) – The astigmatic change by magnitude and axis the surgery intended to induce.
- Surgically-induced astigmatism (SIA) – The amount and axis of astigmatic change the surgery actually induced.
- Magnitude of error (ME) – The arithmetic difference between the magnitudes of SIA and TIA. This is positive for overcorrections and negative for undercorrections.
- Angle of error (AE) – The angle described by the vectors of the achieved correction (SIA) versus the intended correction (TIA). The AE is positive if the achieved correction was on an axis counterclockwise (CCW) to where it was intended and negative if the achieved correction was clockwise (CW) to its intended axis.
- Difference vector (DV) – The vectorial “difference” between the TIA and SIA vectors. It is an absolute measure of success and should ideally be zero.
- Correction index (CI) – The SIA divided by the TIA. The CI is preferably 1.0. It is greater than 1.0 in overcorrection and less than 1.0 in undercorrection.
- Index of success (IOS) – The DV divided by the TIA. The IOS is also a relative measure of success and should ideally be zero.
- Flattening effect (FE) – The amount of astigmatism reduction achieved by the effective proportion of the SIA at the intended meridian.
- Flattening index (FI) – The FE divided by the TIA. This should ideally be 1.0.
- Torque – The amount of astigmatic change induced by the SIA due to nonalignment of the treatment that has been ineffective in reducing astigmatism at the intended meridian but causes rotation and a small increase in the existing astigmatism.
- Refractive predictability – Operationally defined in this study as achievement of attempted correction within ± 0.50 D.
The parameters of vector analysis results were compared between the femtosecond-LASIK and SMILE groups. For each treatment group, four standard vector graphs (TIA, SIA, CI, and DV) were created using the astigmatic software by Gauvin and Wallerstein. Vector graphs were made in the form of single-angle polar plots.13
Visual and Refractive Outcomes
The following parameters were collected at 3 postoperative months: mean postoperative LogMAR UDVA, mean postoperative SE, and mean postoperative cylinder. Parameters 1, 2 and 3, 4 were used to assess the visual and refractive outcomes.14
- Uncorrected Distance Visual Acuity (Efficacy) – The cumulative postoperative uncorrected distance visual acuity (UDVA) three months after femtosecond-LASIK or SMILE graphed in comparison with the cumulative preoperative CDVA.
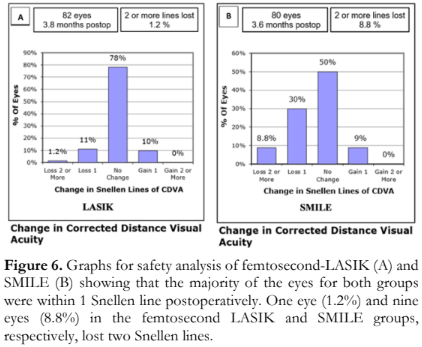
- Safety – The change in preoperative and postoperative CDVA in terms of gain or loss of lines of Snellen visual acuity three months after femtosecond-LASIK or SMILE. A difference of two Snellen lines worse from preoperative CDVA was considered significant.
- Refractive predictability in terms of spherical equivalent – The comparison of the attempted and the actual spherical equivalent (SE) refraction three months after femtosecond-LASIK or SMILE.
- Refractive predictability in terms of cylindrical correction – The comparison of the target- induced astigmatism and surgically- induced astigmatism three months after femtosecond-LASIK or SMILE.
Analyses of refractive and visual outcomes were done using Microsoft Excel 2016 with the nine standard graphs for reporting outcomes of refractive surgery.12 The predictability graphs showed equation trend lines wherein a slope value (m) close to 1 and an intercept value (c) close to zero connote more accurate results. The coefficient of determination (R2) indicated how strong the correlation was between the attempted and achieved correction with a stronger correlation for values closer to 1.
Statistical Analysis
In comparing the two treatment groups, Student t-test was utilized for continuous variables and Chi-square test for categorical variables. The level of significance was set at 5%. MedCalc Statistical software (MedCalc Software Ltd, Belgium) was used to carry out the statistical calculations.
RESULTS
There were 82 femtosecond-LASIK-treated eyes and 80 SMILE-treated eyes included in the study. The clinical profile is presented in Table 1. The mean age of patients was 30.7 ± 8.7 and 31.2 ± 5.9 years in the femtosecond-LASIK and SMILE groups, respectively (p=0.72). The proportion of male patients was similar in both groups (p= 0.73). The mean preoperative sphere was lower for the femtosecond-LASIK group ( -4.2 ± 2.4 D versus -4.9 ± 1.6 D, [p=0.03]) with a similar mean preoperative cylinder (-1.5 ± 0.6 D versus -1.4 ± 0.7 D, [p=0.43]) for the two groups. The mean follow-up time was 3.6 months and 3.8 months for the femtosecond-LASIK and SMILE groups, respectively (p=0.08).

Table 2 compares the different postoperative vector parameters using the Alpins method. There were no significant differences in the mean TIA and SIA cylinder values between femtosecond- LASIK and SMILE groups (p=0.54 and p=0.20, respectively). The mean DV was significantly lower at 0.1 ± 0.2 D in the femtosecond-LASIK group vs 0.3 ± 0.3 D in the SMILE group (p=0.00). The femtosecond LASIK-treated eyes had a significantly lower mean IOS of 0.1 + 0.2 compared to 0.3 + 0.2 in SMILE-treated eyes (p=0.00). The femtosecond-LASIK group also had a significantly lower torque compared to the SMILE group (0.0 + 0.01 vs 0.2 + 0.2, respectively [p=0.00]). The mean FI in the femtosecond-LASIK group was closer to 1.0 than that of the SMILE group (1.0 + 0.1 vs 0.9 + 0.2, respectively [p=.01]). On the other hand, the means of AE, ME and CI were similar in both groups (p=0.06, 0.07, 0.06, respectively). Overall, vector parameters showed better outcomes for DV, IOS, torque, and FI in femtosecond LASIK compared to SMILE.

Figures 1 and 2 show the single-angle polar vector graphs for the femtosecond-LASIK-treated and SMILE-treated eyes, respectively. The four boxes individually plot the mean and individual vector results of TIA, SIA, DV and CI. The blue, red, and white-shaded areas in the map represent with the rule (WTR), against the rule (ATR) and oblique astigmatism, respectively. The red diamonds indicate the vector mean position. The vector mean values are displayed in the call-out boxes along with the arithmetic mean or geometric mean. Each vectorial point is plotted as a black line with a blue circle marker at the end.
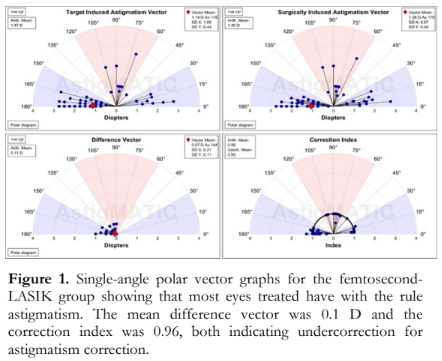

In both groups, the majority of eyes had WTR astigmatism correction as seen in the blue- shaded areas of the graphs. The DV graph shows the remaining astigmatism confirming that femtosecond-LASIK-treated eyes had lower residual astigmatism (mean of 0.1 ± 0.2) compared to SMILE-treated eyes (0.3 ± 0.3) [p=0.00]. The CI graphs also show that the majority of eyes were undercorrected, manifesting as vector points below the black arc plotted at the index value of 1. No significant difference was found comparing the CI of both groups (1 ± 0.1 vs 0.9 ± 0.2; p= 0.06) [Table 2].
Table 3 compares the postoperative visual and refractive outcomes between the femtosecond-LASIK and SMILE groups. The mean postoperative LogMAR UDVA was significantly better in the femtosecond-LASIK treated eyes (0.006 ± 0.06 LogMAR vs 0.06 ± 0.09 LogMAR, p=0.00). Postoperative mean SE was similar in both groups with -0.17 ± 0.26 D and – 0.10 ± 0.35 D for the femtosecond-LASIK and SMILE groups, respectively (p=0.20). The mean postoperative cylinder was significantly lower for the femtosecond LASIK group (0.11 ± 0.22 D vs 0.32 ± 0.30 D, p=0.00).
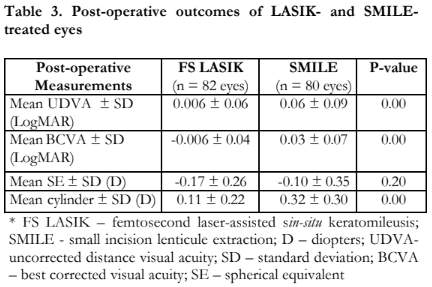
Refractive predictability of femtosecond- LASIK and SMILE in terms of spherical refraction showed good predictability for both groups with the equation of trend lines indicating a slope close to 1 (femtosecond LASIK 0.9764 vs SMILE 0.9806) and a y-intercept close to zero (femtosecond LASIK +0.0062 vs SMILE -0.001) (Figure 3). A strong correlation was also noted between the attempted and achieved spherical equivalent correction of both groups (R2 of 0.9976 and 0.995 for the femtosecond-LASIK and SMILE, respectively).
Refractive predictability in terms of cylindrical correction showed good predictability for low cylinder treatments with y-intercepts close to zero (femtosecond-LASIK: 0.0052 and SMILE: -0.0022) [Figure 4]. However, both showed a trend towards undercorrection when dealing with higher cylinder values, resulting in a slope of 0.9418 in femtosecond-LASIK group and 0.9083 in SMILE group. Strong correlations were noted between the overall TIA and SIA in both groups (R2 of 0.9775 and 0.9489 for femtosecond-LASIK and SMILE groups, respectively).

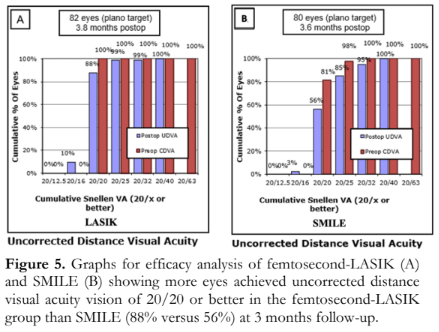
Figure 5 shows treatment efficacy. The femtosecond-LASIK and SMILE groups had relatively similar proportions of eyes with a postoperative UDVA of 20/32 or better after 3 months (99% versus 95%). However, the femtosecond-LASIK group showed a higher proportion of eyes with a postoperative UDVA of 20/20 or better after 3 months of treatment (88% versus 56%).
In terms of treatment safety, a total of 98.8% and 91.2% of the eyes in the femtosecond-LASIK and SMILE groups, respectively, gained lines of Snellen visual acuity or lost not more than one 1 line of Snellen VA (Figure 6). One eye (1.2%) and 9 eyes (8.8%) in the femtosecond-LASIK and SMILE groups, respectively, lost two Snellen lines. A gain of 1 line was seen in 10% of the eyes in the femtosecond-LASIK group and 9% of the eyes in the SMILE group.
DISCUSSION
There are several studies comparing the clinical outcomes of LASIK and SMILE showing contradictory results, with most studies showing no difference in terms of astigmatic correction between the two treatment modalities.3,4,6,9,10,15,16 One study demonstrated that SMILE was inferior to femtosecond-LASIK in terms of astigmatic correction.17 Inconsistencies in the results may be due to different LASIK techniques employed in the different studies. In contrast, all studies used the same machine for SMILE.
In this study, we specifically compared the astigmatic correction of femtosecond-LASIK and SMILE in eyes with -0.75 to -3.0 D of astigmatism. Our study findings showed that the mean postoperative residual cylinder was significantly lower with the femtosecond-LASIK group versus the SMILE group (0.11 ± 0.22 D versus 0.32 ± 0.30 D, p=0.00). This was supported by the lower DV value, IOS value closer to 0, lower torque, and FI value closer to 1.0 in the femtosecond-LASIK group (Table 2). The single angle polar vector graphs (Figures 1 and 2) also showed lower postoperative astigmatism or DV in the femtosecond-LASIK group compared to SMILE-treated eyes. All these findings may be due to treatment misalignment in SMILE, since the machine does not have an eye-tracking capability and a cyclotorsion-compensation system.18,19 As many as 82% of patients exhibit ocular cyclotorsion on supine position.18 To address this, manual compensation has been suggested for SMILE. Limbal markings at 0 and 180 degrees can be made while the patient is in the upright position.18
This study also found a higher proportion of patients achieving UCVA of 20/20 or better with femtosecond-LASIK versus SMILE after 3 months. In terms of safety, both procedures showed high safety with the majority of eyes exhibiting postoperative CDVA unchanged from preoperative CDVA or CDVA loss of not more than 1 Snellen line. However, femtosecond-LASIK was superior, as seen in the 1.2% of patients in the LASIK group losing 2 or more Snellen lines of VA, compared to 8.8% of patients in the SMILE group. These efficacy and safety findings may be accounted for by the better astigmatic correction for low to moderate astigmatism in femtosecond-LASIK and the postoperative healing pattern in SMILE. Additional surgical manipulation for the removal of the lenticule after the laser cut has been suggested to cause topographic irregularities in a small proportion of eyes after SMILE.4,20 This is in contrast with LASIK where the irregularities in the stromal bed after LASIK flap creation are smoothened by the excimer laser.
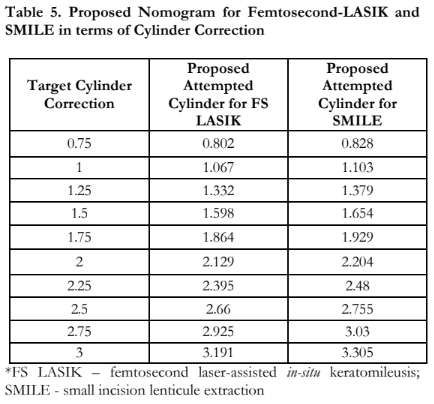
This study also demonstrated that both femtosecond-LASIK and SMILE produced predictable results for the correction of low to moderate myopic astigmatism. However, there was a greater trend for undercorrection in higher degrees of astigmatism in SMILE than in femtosecond-LASIK (Figures 3 and 4). This study is similar to other studies that reported more undercorrection for moderate astigmatism with SMILE.16, 20-22 Ivarsen et al. reported a 13% per diopter undercorrection in a low astigmatism correction attempt, and a 16% per diopter undercorrection in a high astigmatism correction attempt after SMILE.20 Given the results of this study, we have formulated a proposed nomogram based on the spherical equivalent and cylindrical correction of both femtosecond-LASIK and SMILE. Table 4 demonstrates the proposed attempted SE to achieve the target SE. Based on the nomogram, a SE of at least -9.5 D for femtosecond-LASIK would need an adjustment of 0.23 D whereas a SE of at least -10.0 D for SMILE would need an adjustment of 0.20 D. Table 5 demonstrates the proposed attempted cylinder settings to be entered into the machine to achieve the target cylindrical correction. Based on the nomogram, an astigmatism of 2.5 D would need an additional adjustment of 0.255 D to achieve emmetropia and to overcome the undercorrection in SMILE. For femtosecond-LASIK, astigmatism of 3 D will need 0.191 D of cylindrical adjustment to achieve emmetropia in terms of astigmatism. However, this nomogram should only be used if other factors, such as cyclotorsion, torque, and head misalignment, have been taken into consideration. It is also important to take note that this nomogram is based on an average of 3 months postoperative follow-up.

This study is limited by its retrospective study design, short post-operative follow-up period, unequal distribution of magnitudes of astigmatism, and involvement of multiple refractive surgeons. A possible source of bias in the treatment outcomes for SMILE is the number of surgeons (six) performing the procedures, as treatment centration is surgeon-dependent. It is recommended that future studies have a longer follow-up period, since the stability of refraction on longer follow-up can be a more reliable basis for the nomogram. Subgroup analysis according to the magnitude of astigmatism is also suggested. Finally, a comparative analysis of visual and refractive outcomes for astigmatism correction utilizing newer technology (i.e. Visumax 800 and MEL 90, Carl Zeiss Meditec, Germany) is also recommended.
Overall, this study demonstrated that at 3 months, femtosecond-LASIK had superior astigmatic outcomes compared to SMILE in terms of the residual postoperative astigmatism, difference vector, index of success, torque, and flattening index. Despite achieving good visual and refractive outcomes, the tendency for undercorrection is higher in SMILE compared to femtosecond-LASIK when treating higher degrees of astigmatism.
ACKNOWLEDGMENTS
The authors would like to thank the St. Luke’s Medical Center Tan Eng Gee Eye Institute for granting access to data needed for the research analysis. We also thank St. Luke’s Medical Center Research and Biotechnology Division for providing support, approval, and assistance on statistical analyses.
REFERENCES
- Holden AB, Fricke TR, Wilson DA, et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016;123:1036-1042.
- Joffe SN. The 25th Anniversary of Laser Vision Correction in the United States. Clin Ophthalmol. 2021;15:1163–117.
- Ganesh S, Gupta R. Comparison of Visual and Refractive Outcomes Following Femtosecond Laser- Assisted LASIK with SMILE in Patients with Myopia or Myopic Astigmatism. J Refractive Surgery. 2014;30(9):590- 596.
- Chan TCY, Wang Y, Ng ALK, et al. Vector Analysis of High (>3 diopters) Astigmatism Correction Using Small-Incision Lenticule Extraction and Laser In Situ Keratomilieusis. J Cataract Refract Surg. 2018 Jul;44(7):802-810.
- Taneri S, Kiefler S, Rost A, et al. Small incision lenticule extraction for the correction of myopic astigmatism. J Cataract Refract Surg. 2019;45:62-71.
- Zhang J, Wang Y, Chen X. Comparison of Moderate to High-Astigmatism Corrections Using Wavefront- Guided Laser In Situ Keratomilieusis and Small-Incision Lenticule Extraction. Cornea. 2016 Apr;35(4):523–530.
- Alió del Barrio JL, et al. Small Incision Lenticule Extraction (SMILE) in the Correction of Myopic Astigmatism: Outcomes and Limitations – An Update. Eye Vis (Lond). 2017;4:26.
- Reinstein DZ, Gobbe M, Gobbe L, et al. Optical zone centration accuracy using corneal fixation-base SMILE compared to eye-tracker femtosecond laser-assisted LASIK for myopia. J Refract Surg. 2015;31:586-92.
- Kobashi H, Kamiya K, Ali MA, et al. Comparison of Astigmatic Correction after Femtosecond Lenticule Extraction and Small-Incision Lenticule Extraction for Myopic Astigmatism. PLoS One. 2015;10(4)e0123408.
- Khalifa MA, Ghoneim AM, Shaheen MS, Piñero DP. Vector Analysis of Astigmatic Changes After Small- Incision Lenticule Extraction and Wavefront-guided Laser In Situ Keratomilieusis. J Cataract Refract Surg. 2017 Mar;43:819-824.
- Han T, Xu Y, Han X, et al. Three year outcomes of small incision lenticule extraction (SMILE) and femtosecond laser assisted in situ keratomileusis (FS-LASIK) for myopia and myopic astigmatism. Br J Ophthalmology. 2019;103:565-568.
- Alpins N. Astigmatism Analysis by the Alpins Method. J Cataract Refract Surg. 2001 Jan; 27(1):31-49.
- Gauvin M, Wallerstein A. AstigMATIC: an automatic tool for standard astigmatism vector analysis. BMC Ophthalmol. 2018 Sep 21;18(1):255.
- Reinstein DZ, Waring GO III. Graphic Reporting Outcomes of Refractive Surgery. J Refract Surg. 2009;25:975–978.
- Lau YT, Shih KC, Tse RH, et al. Comparison of Visual, Refractive and Ocular Surface Outcomes Between Small Incision Lenticule Extraction and Laser-Assisted In Situ Keratomileusis for Myopia and Myopic Astigmatism. Ophthalmol Ther. 2019;8(3):373-386.
- Kanellopoulos AJ. Topography-guided LASIK versus small incision lenticule extraction (SMILE) for myopia and myopic astigmatism: a randomized, prospective, contralateral eye study. J Refract Surg. 2017;33:306–312.
- Chan TC, Ng AL, Cheng GP, et al. Vector Analysis of Astigmatic Correction After Small- Incision Lenticule Extraction and Femtosecond-assisted LASIK for Low to Moderate Myopic Astigmatism. Br J Ophthalmol. 2016 Apr;100(4):553-9.
- Ganesh S, Brar S, Pawar A. Results of intraoperative manual cyclotorsion compensation for myopic astigmatism in patients undergoing small incision lenticule extraction (SMILE). J Refract Surg. 2017;33:506– 512.
- Chow, S, Chow, L, Lee, C, Chan T. Astigmatism Correction using SMILE. Asia Pac J Ophthalmol 2019;8:391-396.
- Ivarsen A, Asp S, Hjortdal J. Safety and complications of more than 1500 small-incision lenticule extraction procedures. Ophthalmology. 2014;121:822–8.
- Pedersen IB, Ivarsen A, Hjortdal J. Changes in astigmatism, densitometry, and aberrations after SMILE for low to high myopic astigmatism: a 12- month prospective study. J Refract Surg. 2017;33:11–17.
- Ivarsen A, Hjortdal J. Correction of myopic astigmatism with small incision lenticule extraction. J Refract Surg. 2014;30:240–247.

