In Vitro Antimicrobial Susceptibility of Common Bacterial Keratitis Pathogens to Topical Ophthalmic Fluoroquinolones
Arabella R. Mendoza, MD, Eleonore B. Iguban, MD
Department of Ophthalmology, Rizal Medical Center, Philippines
Correspondence: Arabella R. Mendoza, MD, DPBO
Office Address: Rizal Medical Center, 425 Pasig Boulevard, Pasig City, Philippines
Office Phone Number: +639279873613
Email: arramendozamd22@gmail.com
Disclosures: The authors report no conflict of interest.
Bacterial keratitis is a common sightthreatening condition. If left untreated, it often leads to progressive tissue destruction with corneal perforation or extension of infection to adjacent tissues. Common predisposing factors include contact lens wear, trauma, contaminated ocular medications, impaired defense mechanisms, and altered structure of the corneal surface.1 Clinical manifestations of bacterial keratitis are sudden onset of pain accompanied by conjunctival injection, photophobia, and decreased vision. Analysis of 2,064 microbial keratitis cases seen at the External Eye Disease Clinic of the Department of Ophthalmology of the Philippine General Hospital from 1972 to 1996 showed that the most common bacterial organisms isolated were Streptococcus pneumoniae (24.4%), Pseudomonas aeruginosa (14.9%) Moraxella sp. (9.8%), and Staphylococcus aureus (4.1%).2
The recommended treatment for bacterial keratitis is a broad-spectrum topical ophthalmic antibiotic. Fluoroquinolones are good options as they possess broad activity against Gram-positive and Gram-negative bacteria with good safety profile.3 Fluoroquinolones inhibit enzymes involved in bacterial DNA synthesis called DNA gyrase enzymes, also known as topoisomerase II and topoisomerase IV. Second-generation fluoroquinolones ciprofloxacin and ofloxacin are widely used in treating bacterial keratitis. They have great potency against Gram-negative bacilli including P. aeruginosa, moderate activity against S. aureus, and minimal activity against Streptococcus pneumoniae.4 More advanced molecular modifications of the fluoroquinolones in the year 2000 led to the development of the third-generation levofloxacin and fourth-generation moxifloxacin and gatifloxacin.5 Several pharmacokinetic studies have shown that moxifloxacin with its increased lipophilicity has better corneal penetration compared with other fluoroquinolones.
This study aimed to determine the in vitro susceptibility of three common bacterial isolates to innovator and locally manufactured topical ophthalmic fluoroquinolones available in the Philippine market. It also compared the efficacy of the locally manufactured topical ophthalmic fluoroquinolones with their innovator brand counterparts. Prior to this study, there were no reports in literature comparing the antimicrobial effectiveness of locally manufactured topical ophthalmic fluoroquinolones versus their innovator brands.
Moreover, when this study was conducted, ofloxacin was the only topical ophthalmic fluoroquinolone medication included in the Philippine National Drug Formulary (PNDF). This study may provide support for the inclusion of other topical ophthalmic fluoroquinolones in the formulary.
METHODS
This study was a single-masked, experimental study that compared the in vitro susceptibility of S. aureus, S. pneumoniae, and P. aeruginosa to 11 commercially available topical ophthalmic fluoroquinolones, specifically: besifloxacin 0.6% (Besivance, Bausch and Lomb Inc., USA), ciprofloxacin 0.3% (Ciloxan, Novartis Pharma AG, Basel, Switzerland; and Celsus ciprofloxacin, E.L Laboratories, Inc., Philippines), ofloxacin 0.3% (Ofbeat, Synergen Asia, Singapore; and Inoflox, Unilab Inc., Philippines), gatifloxacin 0.3% (Zymar, Allergan, Chicago, Illinois, USA; and Agatiflox, Sensomed Phils, Philippines), moxifloxacin 0.5% (Vistamox, Vista Pharma Inc., Philippines; and Vigamox, Novartis Pharma AG, Basel, Switzerland), and levofloxacin (Oftaquix, Santen Pharmaceutical Co. Ltd., Japan; and Leeflox, Centaur Pharmaceuticals Pvt Ltd., India). Zones of inhibition were recorded for each of the topical fluoroquinolones being tested.
The research study was conducted at the Microbiology section of the Research Institute of Tropical Medicine Laboratory in Alabang, Muntinlupa.
Preparation of Bacterial Isolates
Laboratory-grown, pure, standard bacterial isolates of S. aureus (ATCC 25923), S. pneumoniae (ATCC 49619), and P. aeruginosa (ATCC 27853) were obtained from the American Type Culture (ATC) collection to avoid resistance patterns and were grown in trypticase soy broth. They were separately inoculated on sterile blood agar plates and were incubated at 35-37 degrees Celsius for 24 hours. To verify the purity of the isolates, a Gram stain was done, and the organism grown was identified. A saline solution of isolated colonies selected from a 24-hour agar plate was used to prepare the inoculum. Using a densitometer, the inoculum suspension was adjusted to match the 0.5 McFarland turbidity standard.
Inoculation of the Test Plates
After adjusting the turbidity of the inoculum suspension, a sterile cotton swab was dipped into the adjacent suspension. The dried surface of a Mueller Hinton agar plate was inoculated by streaking two or more times rotating the plate approximately 60 degrees each time to ensure an even distribution of the inoculum. The isolates were proven to be pure and were evenly swabbed on the Mueller Hinton agar plate. S. pneumoniae was planted in a Mueller Hinton agar plate with 5% sheep’s blood.
Preparation of Topical Fluoroquinolone
One bottle of each topical ophthalmic fluoroquinolone being tested was obtained. The bottles were new, sealed, not tampered, and had the latest manufacturing date. They were used before their expiration dates. Five micrograms of each antibiotic were instilled on separate wafers of filter paper with a diameter of 6mm each.
Preparation of Culture Media
Mueller Hinton agar plates were used for the antimicrobial testing. Using the Kirby Bauer technique of antimicrobial susceptibility testing, the test was done in triplicate. The agar plates were incubated for 24 hours at 35-37 degrees Celsius. A filter paper which was not soaked with topical ophthalmic fluoroquinolone served as the negative control. The negative control filter papers and the filter papers impregnated with 5 micrograms of fluoroquinolone were then placed on their corresponding areas on the agar plates. Big letters were used to label the bacterial isolates and small letters for the filter paper soaked with the antibiotics that were tested (Table 1).
Zone of Inhibition Measurement and Data Analysis
Zones of inhibition were measured using a caliper under reflected light and were corrected in millimeters. Antimicrobial sensitivity of test organisms to the fluoroquinolones were interpreted using the Clinical Laboratory Standards Institute (CLSI) M100-S25 Performance Standards for Antimicrobial Susceptibility Testing tables which showed the recommended breakpoints for zones of inhibition values for various flouroquinolones.6
Statistical Analysis
One-way ANOVA was used to determine significant differences in antimicrobial sensitivity among treatment groups. T-test was used to compare the significant differences between the two brands of each kind of fluoroquinolone. Data were exported to SPSS (Statistical Package for the Social Sciences version 18.5). A p-value of <0.05 was considered significant.
RESULTS
The study demonstrated that P. aeruginosa, S. pneumoniae, and S. aureus were sensitive to all the topical ophthalmic fluoroquinolones tested.
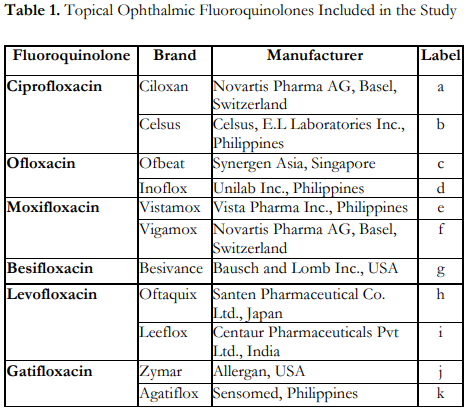
Figure 1 shows the mean zones of inhibition measured from the in vitro susceptibility testing of the 3 bacterial isolates with 11 topical ophthalmic fluoroquinolones. For P. aeruginosa, both brands of ciprofloxacin, Ciloxan and Celsus, had the largest zones of inhibition, while besifloxacin (Besivance) had the smallest zone of inhibition compared to the rest of the topical fluoroquinolones. On the other hand, for S. pneumoniae, moxifloxacin (Vigamox) had the largest zone of inhibition while ciprofloxacin (Ciloxan), ciprofloxacin (Celsus), ofloxacin (Ofbeat), and ofloxacin (Inoflox) had the smallest zones of inhibition. For S. aureus isolates, moxifloxacin (Vigamox) showed the greatest zone of inhibition while ofloxacin (Ofbeat) and ofloxacin (Inoflox) had the smallest zones of inhibition compared to the rest of the topical ophthalmic fluoroquinolones.
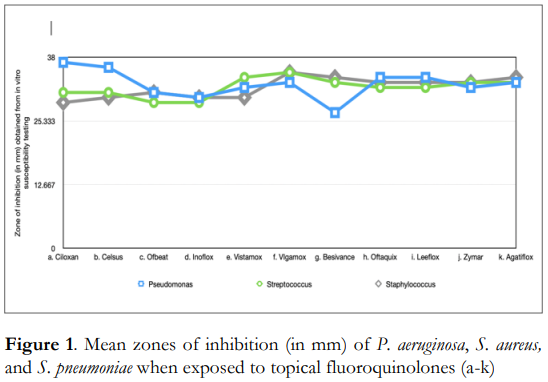
Table 2 shows the zones of inhibition measured from the in vitro susceptibility testing of P. aeruginosa with 11 topical ophthalmic fluoroquinolones. Results of one-way ANOVA showed that there were significant differences in the antimicrobial effectivity of the topical fluoroquinolones tested against the said organism. Meanwhile, t-test results comparing the antimicrobial activity of the innovator versus the locally manufactured brand revealed that the innovator brand of ciprofloxacin, Ciloxan, demonstrated significantly better antimicrobial activity towards P. aeruginosa compared to the locally manufactured brand, Celsus (p=0.001).
Table 3 shows that all the topical fluoroquinolones had significant antimicrobial activity against S. aureus since the measured zones of inhibition were found to meet the cut-off values for sensitivity as based in the CLSI M100-S25 Performance Standards for Antimicrobial Susceptibility Testing tables.6 However, results of one-way ANOVA showed significant differences in the mean zones of inhibition of the topical fluoroquinolones tested against S. aureus. Moreover, the innovator brand of moxifloxacin, Vigamox, demonstrated significantly better antimicrobial activity towards S. aureus compared to the locally manufactured brand, Vistamox.
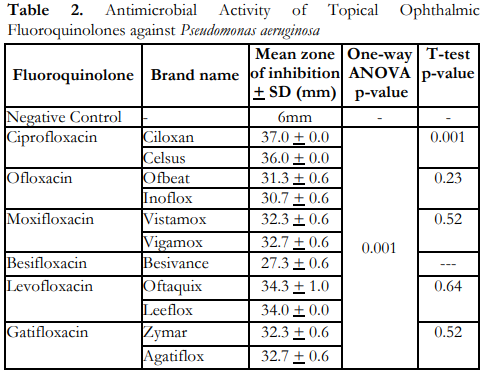
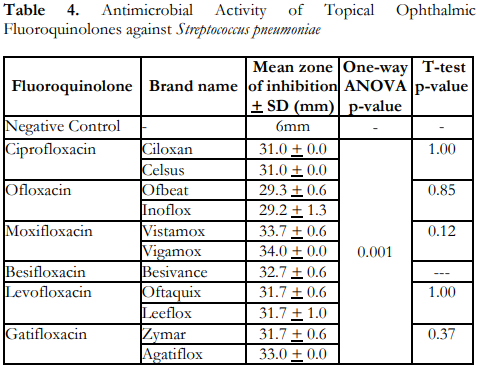
Table 4 shows that all the topical ophthalmic fluoroquinolones demonstrated significant antimicrobial activity against S. pneumoniae based on the CLSI M100-S25 Performance Standards for Antimicrobial Susceptibility Testing tables.6 There were also statistically significant differences in the mean zones of inhibition of the tested flouroquinolones. The innovator brands and the locally manufactured ones did not show any statistically significant difference in terms of the measured zones of inhibition for S. pneumoniae.
DISCUSSION
In vitro susceptibility testing of P. aeruginosa, S. pneumoniae, and S. aureus against the wide range of topical fluoroquinolones available in the Philippine market showed that all the medications tested had significant antimicrobial activity based on the measured zone of inhibition.
For P. aeruginosa, all topical fluoroquinolones showed significant antimicrobial sensitivity, with the largest zone of inhibition seen with both brands of ciprofloxacin: Ciloxan and Celsus. This was consistent with the results of multiple studies which showed that ciprofloxacin was the most effective flouroquinolone against P. aeruginosa, with typical minimum inhibitory concentrations (MICs) one-half to one-eighth of those of the newer generation fluoroquinolones such as levofloxacin, moxifloxacin, and gatifloxacin.7 Similar susceptibility findings were also observed with other Gram-negative bacteria, such as Escherichia coli and Klebsiella pneumoniae.7 Moreover, in an in vitro study conducted by Moore et al. where four fluoroquinolones, namely ciprofloxacin, levofloxacin, ofloxacin, and trovafloxacin were tested on 100 isolates of P. aeruginosa, ciprofloxacin was noted to be the most efficacious in terms of antimicrobial activity.8
Conversely, for Gram-positive organisms, S. pneumoniae and S. aureus, less antimicrobial susceptibilities were observed when exposed to the second-generation fluoroquinolones, ciprofloxacin and ofloxacin. Moxifloxacin, especially its innovator brand Vigamox, produced the largest zones of inhibition as compared to other topical ophthalmic fluoroquinolones tested. This finding was consistent with the study by Duggirala et al. which reported that fourth-generation fluoroquinolones provided greater antibacterial activity against Gram-positive isolates and had greater value in the treatment of ocular infections caused by Gram-positive bacteria.9 Several pharmacokinetic studies have also shown that moxifloxacin has greater corneal penetration compared to other fluoroquinolones, which may explain its superior efficacy.10 Moreover, multiple in vitro studies have also demonstrated that moxifloxacin and gatifloxacin were significantly more potent than levofloxacin against Grampositive organisms.10 These support our finding of Gram-positive organisms being more sensitive to moxifloxacin compared to the other topical ophthalmic fluoroquinolones. However, a systematic review by Bispo et al. showed that there was a high rate of in vitro resistance among S. aureus and coagulase-negative staphylococci (CoNS) to fluoroquinolones, and high rates of occurrence of methicillin-resistant staphylococci.11 This increasing occurrence of antibiotic resistance may have been brought about by empiric treatment with broadspectrum antibiotics without the benefit of culture and sensitivity results. Thus, judicious use of topical ophthalmic antibiotics, guided by culture and sensitivity studies, and continued monitoring of antibiotic sensitivity data should be encouraged to avoid antibiotic resistance and emergence of various resistant ocular microorganisms.
Another prevailing concern is the lack of certain topical ophthalmic antibiotics in some areas of the country. Currently, access to topical antibiotics at the community level is largely influenced by cost and local market availability. Although most innovator topical fluoroquinolone brands are available in city-based pharmacies, the prices are usually higher than the generic or locally manufactured ones. Despite the affordability of the latter, drug regulatory agencies have established regulations that ensure the bioequivalence of generic or locally manufactured topical medications to their respective branded or innovator drug counterparts.12 Therefore, it can be inferred that the locally manufactured topical ophthalmic fluoroquinolones will demonstrate antimicrobial activities which are almost similar to those of the corresponding innovator brands. This was evident in the findings of this study which showed that the tested pathogens are all sensitive to both innovator brands and local brands of flouroquinolones. However, there are significant differences in antimicrobial effect between ciprofloxacin (Ciloxan) and ciprofloxacin (Celsus), as well as moxifloxacin (Vigamox) and moxifloxacin (Vistamox) against P. aeruginosa and S. aureus, respectively.
Despite the advantages brought about by the availability of generic medicines, extensive and comparative data on their clinical equivalence to the innovator brands are still limited.13 The antimicrobial sensitivity differences observed in this study between the two brands of ciprofloxacin (Ciloxan and Celsus), as well as between the two brands of moxifloxacin (Vigamox and Vistamox), may be attributed to minute differences or irregularities in their pharmaceutical or physicochemical properties which could eventually translate to a modified pharmacokinetic and/or pharmacodynamic behavior of the medication.14 Alterations in drug manufacturing standards, brought about by human or machine errors and varied environmental conditions, may also contribute to differences in drug effectivity. In addition, compared to innovator brands, the approval of generic or locally manufactured topical ophthalmic drugs does not require robust and extensive clinical studies on microbial and clinical effectivity prior to market availability.12 Instead of requiring further clinical studies, drug regulatory agencies presume that the bioequivalence of the active ingredient in locally manufactured or generic medications would translate to therapeutic or clinical similarity with innovator brands.15 There are also, unfortunately, very few non-inferiority trials comparing the effectivity of these ophthalmic antibiotic formulations.
Since this was an in vitro study, the use of laboratory grown isolates may not reflect drug resistance patterns in the real world. The authors recommend that additional studies be conducted on actual patient populations to further assess the efficacy of these topical fluoroquinolones. Also, use of isolates obtained from ocular specimens could also be done to mimic actual clinical scenarios which may be more predictive of clinical response in the real-world setting.
REFERENCES
- Feder R, Berdy G, Luorno J, et al. Microbial and Parasitic Infections of the Cornea and Sclera. In: Reidy J ed. Basic Clinical and Science Course. Section 8 External Disease and Cornea. San Francisco: AAO, 2012-2013; chap. 5: 158-162
- Valenton MJ. Central microbial keratitis: etiology, epidemiology, clinical features, treatment. Philipp J Ophthalmol. 2000;25 (Suppl):7-126.
- Baum J, Barza M. The evolution of antibiotic therapy for bacterial conjunctivitis and keratitis: 1970–2000. Cornea. 2000;19(5):659–672.
- Seuke H, et al. New Developments in Antibacterial Chemotherapy for Bacterial Keratitis. In Corneal Disease: Recent Developments in Diagnosis and Therapy, ed. Thomas Reinhard and Frank Larkin, 19-35. New York City: Springer, 2013.
- Sueke H, Kaye S, Neal T. Minimum inhibitory concentrations of standard and novel antimicrobials for isolates from bacterial keratitis. Invest Ophthalmol Vis Sci. 2010;51(5):2519–2524.
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement. CLSI document M100-S25. Jan 1, 2015: https://www.nih.org.pk/wpcontent/uploads/2021/02/CLSI-2020.pdf (accessed February 23, 2024).
- Scheld WM. Maintaining fluoroquinolone class efficacy: review of influencing factors. Emerg Infect Dis. 2003 Jan;9(1):1-9
- Moore E, Swiatlo, E, Watt, J, McDaniel, S. In vitro activity of four fluoroquinolones against clinical isolates of Pseudomonas aeruginosa determined by the E test. Int J Antimicrob Agents. 2000 Jun;15(1):73-6.
- Duggirala A, Joseph J, Sharma S, et al. Activity of newer fluoroquinolones against gram-positive and gramnegative bacteria isolated from ocular infections: an in vitro comparison. Indian J Ophthalmol. 2007;55(1):15– 19.11.
- Scoper S. Review of third and fourth generation fluoroquinolones in ophthalmology: in vitro and in vivo efficacy. Adv Ther. 2008 Oct;25(10):979-94.
- Bispo PJM, Sahm, DA, Asbell, PA, A Systematic Review of Multi-decade Antibiotic Resitance Data for Ocular Bacterial Pathogens in the United States. Ophthalmol Ther. 2022 Apr; 11(2): 503–520.
- Cotia A, Junior H, Matuoka J, Bosczowski I. Clinical Equivalence between Generic versus Branded Antibiotics: Systematic Review and Meta-Analysis. Antibiotics (Basel). 2023 May;12(5):935.
- Tattevin P, Crémieux AC, Rabaud C, Gauzit R. Efficacy and quality of antibacterial generic products approved for human use: a systematic review. Clin Infect Dis. 2014 Feb;58(4):458-69.
- Chow SC, Liu J. Meta-analysis for bioequivalence review. J Biopharm Stat. 1997 Mar;7(1):97-111.
- Raw AS, Furness MS, Gill DS, et al. Regulatory considerations of pharmaceutical solid polymorphism in Abbreviated New Drug Applications (ANDAs). Adv Drug Deliv Rev. 2004 Feb 23;56(3):397-414.

