Subconjunctival injection of bevacizumab for treatment of pterygium
Anthony F. Felipe, MD, Ruben Lim Bon Siong, MD, Harvey S. Uy, MD
PTERYGIUM is a common ocular-surface disorder characterized by the encroachment of a fleshy triangle of
conjunctival tissue into the cornea. In a 30-year (1971 to 2001) survey by the Cornea and External Disease Clinic of the Department of Ophthalmology and Visual Sciences, University of the Philippines–Philippine General Hospital (UP–PGH), pterygium ranked eighth among the 10 leading conditions seen in the clinic and third among the most common noninfectious conditions (Valenton MJ. Clinical presentation and complications of pterygium: the Philippine experience. Unpublished). The pathogenesis of pterygia is not fully understood.1-4 Various studies have implicated environmental factors, such as ultraviolet light, chronic irritation, and inflammation. Recent studies have also provided evidence implicating genetic components, antiapoptotic mechanisms, cytokines, growth factors, extracellular matrix remodeling, immunological mechanisms, and viral
infections in the pathogenesis of the disease.1-5 Histologically, pterygium consists of atrophic conjunctival epithelium and a body of hypertrophic and elastotic degenerated connective tissue.6-8 More recently, tumor-like characteristics have also been found in pterygia. Among them are histologic features such as mild dysplasia, local invasiveness, and high recurrence rate.9 Although histologically benign, pterygia are aggressive in nature and have the propensity to encroach substantially on the ocular surface. They are, thus, capable of causing significant cosmetic deformity and visual impairment. Vascular growth factors, such as vascular endothelial growth factor (VEGF), have been detected in pterygium.10-13 There is marked elevation of VEGF in pterygia compared with normal conjunctival samples.10-13 Although the pathogenesis of pterygia is still poorly understood, their formation and progression are known to depend on neovascularization. It has been postulated that the development of pterygia depends on a changed angiogenic stimulator-to-inhibitor ratio. Jin and colleagues showed that pterygia contain drastically decreased levels of pigment epithelium-derived factor, an angiogenic inhibitor, and elevated VEGF levels.13 Hill and Maske speculated as early as 1989 on the possible role of angiogenic mechanisms in the pathogenesis of pterygia.2 They found that limbal vessels are normally prevented from entering the cornea because of growth-inhibiting substances in the cornea. Neovascularization of the cornea could arise through destruction of the inhibiting substances, with elaboration of substances that stimulate limbal vessels to grow toward the site of maximum concentration. VEGF has been shown to be strongly increased in pterygium and is suggested to be involved directly or indirectly in its pathogenesis.10-13 Bevacizumab (Avastin, Genentech Inc., San Francisco, CA, USA) is a full-length, humanized, monoclonal antibody against all types of VEGF. It binds to and neutralizes the biologic activity of all types of human VEGF, thus preventing its interaction with its receptors on the surface of endothelial cells. It is approved by the United States Food and Drug Administration for the treatment of metastatic colorectal cancer. Added to other chemotherapeutic agents and given intravenously, bevacizumab caused significant improvement in overall survival, progression-free survival, and response rate among patients with metastatic colorectal cancer.14 Together with other anti-VEGF agents, it has been studied for ophthalmic conditions15-17 with abnormal blood-vessel proliferation as the main pathology. Bevacizumab has been used to treat
choroidal neovascularization due to age-related macular degeneration (ARMD), and more recently diabetic macular edema. Various clinical trials have shown that when given intravitreally, it is well tolerated and associated with improvement in visual acuity, decreased central retinal thickness, and reduction in angiographic leakage.18-20
The suggestion of the potential clinical efficacy of intravitreal bevacizumab has led to its widespread use for ARMD. Pterygium is best removed surgically.21 Although recurrence rates can be high in some cases, additional procedures such as conjunctival- or amniotic-membrane transplantation have been developed to improve surgical outcomes.22 In spite of the relative success of these procedures, however, they still present as a challenge to many surgeons. Thus, the search for a safe, effective, and technically simpler form of cure continues. No study has yet been reported or published using subconjunctival injection of an anti-VEGF, such as bevacizumab, as an alternative treatment for pterygium. If elevated VEGF levels contribute to the fibrovasculartissue proliferation seen in pterygia, our hypothesis is that local administration of VEGF inhibitors, such as bevacizumab, will cause a decrease in pterygium vascularity coupled with change in the size of the pterygium. Once validated, this technique may obviate the need for surgery as well as provide an alternative
form of therapy for pterygia that is simpler, faster, and easier to perform compared to existing methods of
pterygium removal.
This study determined the biologic effect and safety of subconjunctival injection of bevacizumab for primary or
recurrent pterygium. More specifically, it sought to:
1. determine any changes in the pterygium in terms of vascularity and thickness within and up to 4 months
postinjection,
2. estimate any change in size of pterygium within and up to 4 months postinjection based on surface area, and
3. describe any postinjection complications and adverse events.
METHODOLOGY
This off-label, multiple-dosing, interventional case series was conducted at the Sentro Oftalmologico Jose
Rizal, UP–PGH. The protocol and informed-consent forms underwent technical and ethical review and were
duly approved by the institution’s ethics review board. The research was done in accordance with the guidelines set by the Declaration of Helsinki. Eligible subjects were at least 18 but not over 40 years
old and diagnosed with primary and recurrent pterygium. Each pterygium was measured and graded according to the grading scheme proposed by Tan and coworkers in 1997.23 Grading was based on the visibility of the underlying episcleral blood vessels. This has been previously described and validated as a marker of severity. The pterygia were classified into grades 1, 2, or 3 based on slitlamp biomicroscopy evaluation. Grade 1 (“atrophic”) had clearly visible episcleral vessels under the body of the pterygium. Grade 2 (“intermediate”) had partially visible episcleral vessels under the body of the pterygium. Grade 3 (“fleshy”) had totally obscured episcleral vessels underlying the body of the pterygium. On baseline examination, the pterygium should have been at least grade 2 or 3 with apex of the lesion midway between the limbus and the pupillary border. If the patient had pterygia in both eyes, the eye with the worse grading was injected. If both eyes had similar gradings, the right eye was injected. Excluded were patients who had undergone any form of ocular surgery except pterygium removal, any condition for which bevacizumab is contraindicated (allergy to bevacizumab, hypertension, proteinuria, bleeding tendencies, previous myocardial infarction or stroke, pregnant and lactating women), evidence of other ocular diseases except error of refraction, administration of topical medications for pterygium, other complaints not attributable to the pterygium, prior ocular trauma, and inability to follow up for the duration of the study.
After eligibility was confirmed and informed consent was obtained, study patients were interviewed by one
investigator (AFF). The off-label use of the drug and its potential risks and benefits were discussed extensively with the patients. All patients were informed of the alternative procedures for pterygium; namely, excision with mitomycin C and conjunctival autograft. The patients were interviewed prior to injection using a questionnaire to obtain information such as general data; contact number; demographic factors; medical, surgical, and ocular history. A complete eye evaluation was performed for each patient. This included Snellen visual-acuity determination, automated tonometry, slitlamp examination, and anteriorsegment photography. Baseline blood pressure was taken using an aneroid sphygmomanometer. At baseline and at all subsequent follow-ups, the
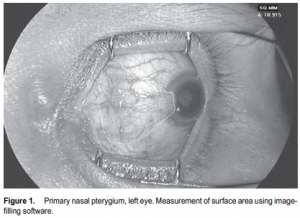
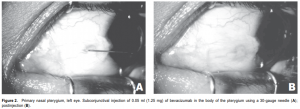
appearance of the pterygium was documented by anteriorsegment photography using a Topcon TRC-50IX Retina Camera (Topcon, Fotan, Hong Kong) at standard magnification (50o ) and distance for anterior-segment photos under controlled lighting conditions. The patients were asked to direct the eye to be injected in extreme horizontal gaze to maximize exposure of the pterygium. The dimensions of the pterygium were determined in the anterior-segment photo by measuring its length in centimeters, from base (using the caruncle as landmark) to apex, and width in centimeters at the base and apical areas. All photos were then uploaded and analyzed for computation of the surface area by an image-filling software, IMAGE J version 1.34s (National Institutes of Health, Bethesda, MD, USA) (Figure 1). Three readings were recorded and the mean was computed. All injections were performed by a single investigator (AFF) under slitlamp biomicroscopy at 10x magnification. After placing topical 0.5% proparacaine hydrochloride (Alcaine, Alcon, Fort Worth, TX, USA), a self-retaining lid retractor was placed and the patient was asked to direct the eye to be injected in extreme horizontal gaze to have adequate exposure of the pterygium. Using a 1-ml syringe with 30-gauge needle, 1.25 mg (0.05 ml) of bevacizumab was injected to the subconjunctival area of the body of the pterygium (Figure 2). Two drops of 0.5% moxifloxacin hydrochloride (Vigamox, Alcon, Fort Worth, TX, USA) were subsequently instilled in the eye. The lid retractor was removed and the patient was sent home with a predetermined follow-up schedule. Injections were repeated every 2 weeks for 10 weeks for a total of 6 injections (total dose of 7.5 mg per patient). The multiple dosing was based on bevacizumab’s half life and temperature sensitivity that makes it easily degraded by body temperature. Patients were followed up every 2 weeks for 10 weeks, then one and a half months after the last injection (total of 7 visits). During each follow-up, a complete ophthalmologic evaluation was performed and the blood pressure was taken. Any postinjection complications and adverse events were noted and attended to. Anterior-segment photography, using the same camera and by the same photographer, was done during each visit. After the followups were completed, the series of anterior-segment photographs were shown to 3 independent masked observers. They were tasked to grade the appearance of each eye using the previously described scale of vascularity and thickness. Postinjection complications, such as ocular surface toxicity, corneal abrasion, persistent epithelial defect, subconjunctival hemorrhage, infection, and uveitis were noted. Any adverse event, defined as any untoward event occurring postoperatively that did not necessarily have a causal relationship with the treatment, was likewise noted.
These may include systemic effects such as hypertension or any thromboembolic incidents, or any allergic reactions to the medications and products used in the study. Throughout the course of the study, all adverse events, including severity, action taken, and relationship to the products and medications used on the patients, were monitored and reported on the adverse-events alert card.
Outcome Measures
The drug was considered to have a biologic effect if it caused any regression in the size of the pterygium based on surface area (in cm2 ) measured, and any decrease in vascularity and thickness based on pterygium grading by 3 masked observers. The primary safety variable monitored was the occurrence of adverse events, especially hypertension and thromboembolic events. Blood pressure was checked during each visit. Other safety variables monitored included Snellen visual acuity, applanation tonometry, and slitlamp biomicroscopy.
Statistical Analysis
Both descriptive and analytic approaches were used in the data analyses. All numerical continuous data were
summarized using descriptive statistics (percentage, frequency distribution, and measures of central tendency). The comparison of the different numerical variables from baseline until after the last follow-up was computed using the repeated measures analysis of variance (ANOVA). Paired t-test for pairwise comparison was performed for significant results. For the grading of pterygia, comparison of the median grading from baseline until after 16 weeks of observation was done using the Friedman test. Pairwise comparison was done using the Wilcoxon signed-rank test. All statistical analysis were carried out using the licensed statistical software Statistical Package for the Social Sciences (SPSS ver. 10). A p value less than or equal to 0.05 was considered statistically significant.
RESULTS
From February 2007 to August 2007, 15 patients (12 males and 3 females) were included in the study. Age
ranged from 23 to 40 years with a mean of 30.13 years (Table 1). There were 14 primary (13 unipolar, 1 bipolar) and 1 recurrent pterygia. There was no statistically significant difference in the mean surface areas of the pterygia at different intervals (p > 0.05) (Table 2). At baseline, mean surface area was 1.22 ± 0.19 cm2
. At weeks 10 and 16 (1 month postinjection), mean surface areas were 1.22 ± 0.18 cm2
and 1.22 ± 0.17 cm2 respectively. The mean surface areas were comparable to baseline until after the sixth injection. Comparing the mean pteygium grading by the 3 masked observers at different intervals, a significant
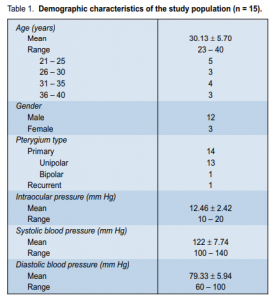
difference was noted (p < 0.01) (Table 2). This difference was noted when the mean grading at baseline was
compared with the mean grading at either the tenth (p = 0.025) or 16th week (p = 0.025). At baseline, there
were 11 (73.3%) patients with grade 2 pterygium and 4 patients (26.7%) with grade 3. At week 8 follow-up, there were 3 (20%) patients with grade 1 pterygium, 7 (46.7%) with grade 2, and 5 (33.3%) with grade 3. At week 16 postinjection (follow-up), there were 5 patients (33.3%) with grade 1 pterygium, 7 (46.7%) with grade 2, and 3 (20%) with grade 3. The 5 patients with grade 1 pterygium at the end of the study period had a baseline grading of 2 from all 3 observers. In terms of visual-acuity changes, comparison of the Snellen visual acuity at different time intervals showed no statistically significant difference (p > 0.05). At baseline,
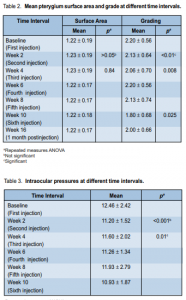
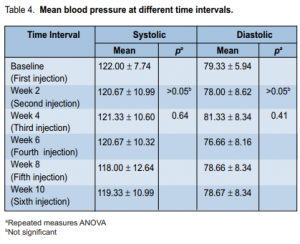
14 (93.3%) patients had Snellen visual acuity of 20/20 while 1 patient had 20/30. At 10-week follow-up, visual acuity of 14 patients (93.3%) was still 20/20. Comparison of cylindrical refraction at different time intervals also showed no significant difference (p > 0.05). There was a statistically significant difference (p < 0.001) in intraocular pressures (IOP) at different time intervals (Table 3).
Pairwise comparison showed that the differences occurred when the baseline IOP was compared with
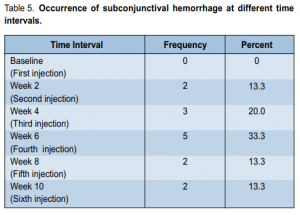
the IOP at either the second (p = 0.027), sixth (p = 0.018), or tenth (p = 0.029) week of observation. At baseline, the IOP ranged from 10 to 20 mm Hg with a mean of 12.46 ± 2.42 mm Hg. At second week, it ranged from 10 to 14 mm Hg with a mean of 11.20 mm Hg. At the fourth, sixth, eighth, and tenth weeks of follow-up, the mean IOP was 11.60, 11.26, 11.93, and 10.93 mm Hg respectively. The mean IOP at the second, sixth, and tenth weeks significantly decreased from baseline. Systolic (SBP) and diastolic (DBP) blood pressure at different time intervals showed no significant difference (p > 0.05) (Table 4). SBP at different intervals from baseline were comparable throughout the study. No ocular-surface toxicity, persistent epithelial defects, corneal abrasion, infections, or uveitis were reported during the study. Occasional subconjunctival hemorrhage was observed after injection and these resolved without intervention within 1 week. The occurrence of subconjunctival hemorrhage at different time intervals is shown in Table 5.
DISCUSSION
VEGF has been shown to be increased in pterygium and is suggested to be either directly or indirectly involved
in its pathogenesis.10-14 Immunohistochemistry studies have shown that VEGF levels are more expressed in pterygium than in normal conjunctiva.9,13 Decreased antiangiogenic factors, together with increased stimulators, have been hypothesized in the formation and progression of pterygia.13 The findings of abundant
expression of VEGF in pterygium may lead to the development of anti-VEGF therapy aimed at inducing regression of blood vessels and size of pterygium. To the best of our knowledge, this is the first study that
has used an anti-VEGF agent as a novel therapy for pterygium. In our series, there was no significant regression in the size of the pterygium after six injections of bevacizumab (p > 0.05). There was no difference in the mean surface area before (1.22 ± 0.19 cm2 ) and after (1.22 ± 0.17 cm2 ) treatment.
Pterygium is a chronic, degenerative disorder described histologically as elastotic degeneration of conjunctival
tissue. It has a stromal overgrowth of fibroblasts and blood vessels accompanied by an inflammatory cell infiltrate and abnormal extracellular matrix accumulation composed of elastin and collagen.1 Although the exact mechanism of pterygium formation is still not fully understood, recent studies have shown that several factors, such as antiapoptotic mechanisms, proinflammatory cytokines and growth factors, extracellular matrix remodeling, immunological mechanisms, viral infections, and a genetic component may be involved in the pathogenesis of this disease.1-5 Given the multiple factors that may affect the natural course of the disease, we presumed that the drug was ineffective because of the interplay of these other mechanisms. Bevacizumab is a full-length, humanized, monoclonal antibody directed against all types of VEGF but not to the other growth factors found in pterygium. The dose of the drug could be too low for a fibrovascular tissue such as pterygium. Theoretically, a very vascular tissue will have a greater degree of systemic absorption of a given drug; thus, limiting its effect locally. During subconjunctival injection, it was also observed that some of the drug flowed out of the needle track and a certain amount was lost every time a patient blinked as the drug was squeezed out of the subconjunctival area. To overcome these factors, we injected the drug every 2 weeks for 10 weeks (total of 6 injections). We used 1.25 mg of bevacizumab based on the dose used in intravitreal
injections. The optimal dose and frequency for subconjunctival use is still undetermined. It is possible that a higher dose and a different dosing schedule for longer periods may be superior to the method we used; however, we chose to err on the side of undertreatment until further toxicity data are obtained. The drug was used mostly on grades 2 and 3 primary pterygia and on recurrent pterygia. These pterygia have thicker and greater amount of fibrovascular tissue compared with those in the early stages.23 There is lack of data regarding the penetration, temporal, and spatial capacity of bevacizumab on ocular-surface diseases like
pterygium. We theorized that bevacizumab was unable to penetrate these tissues. Even if the drug was able to
penetrate the tissue, it is still uncertain whether the dose and half-life of the drug was enough to cause regression in the size of the pterygium. There have been reports on effectiveness of the drug on corneal neovascularization but these tissues have different histological composition.24 In 5 (33.3%) patients, there was note of a decrease in pterygium vascularity and thickness (p < 0.01). Lee and coworkers found that VEGF was strongly expressed in the epithelium of pterygia.11 Philipp and colleagues found that VEGF and its receptors, Flt-1 and Flk-2, were greatly increased in the endothelial cells of newly formed vessels in the stroma of inflamed corneas.14 However, because of the lack of studies on bevacizumab penetration on pterygia, we can only speculate that since all 5 had milder forms of pterygia at baseline (grade 2) with relatively little
neovascularization, the drug was able to penetrate these tissues and induced regression in blood vessels. It is important to note that even though the interobserver variability was low, the gradings were subjective. Until such time that pterygium vascularity and thickness can be objectively quantified, we can only infer clinically that bevacizumab has a potential to cause shrinkage and regression in the vascular caliber of pterygium blood
vessels. Bevacizumab is not indicated for subconjunctival injection, but it seemed to be safe and well tolerated in patients in this study. There was no significant change in mean Snellen visual acuity from baseline (20/20)
(p > 0.05) nor any rise in IOP. There were also no reports of corneal abrasion, ocular-surface toxicity, uveitis,
endophthalmitis, or any thromboembolic events after injection. Eleven (73.3%) patients had at least 1 episode
of mild subconjunctival hemorrhage but this was considered a minor complication and easily resolved in a
week. There were no significant changes in mean systolic and diastolic blood pressures after 10 weeks of observation (p > 0.05). Two (13.3%) patients had occasional blood pressure rises < 140/90 mm Hg, presumably due to preexisting hypertension not detected during the initial evaluation. Though the possibility of systemic side effects still exists, their occurrences were reduced since the amount of drug used was 300- to 400-fold less compared with intravenous infusion. In conclusion, subconjunctival injection of 1.25 mg bevacizumab given every 2 weeks for 10 weeks did not result in significant change in the size of the pterygium. Local application of bevacizumab, however, showed promise in inducing regression in pterygium vascularity and thickness. No serious ocular and systemic adverse events were noted. Controlled clinical trials with more patients and longer observation time are needed to determine its role in modulating angiogenesis in pterygium. Such studies can evaluate the dose and route of administration of bevacizumab in early stages of pterygium and as an adjunct to pterygium surgery.
References
1. Di Girolamo N, Chui J, Coroneo MT, Wakefield D. Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog Retin Eye Res 2004; 23: 195-228.
2. Di Girolamo N, Coroneo MT, Wakefield D. Active matrilysin (MMP-7) in human pterygia: potential role in angiogenesis. Invest Ophthalmol Vis Sci 2001; 42: 1963- 1968.
3. van Setten G, Aspiotis M, Blalock TD, et al. Connective tissue growth factor in pterygium: simultaneous presence with vascular endothelial growth factor—possible contributing factor to conjunctival scarring. Graefes Arch Clin Exp Ophthalmol 2003; 241: 135-139.
4. Solomon A, Grueterich M, Li DQ, et al. Overexpression of insulin-like growth factorbinding protein-2 in pterygium body fibroblasts. Invest Ophthalmol Vis Sci 2003; 44: 573-580.
5. Maini R, Collison DJ, Maidment JM, et al. Pterygial derived fibroblasts express functionally active histamine and epidermal growth factor receptors. Exp Eye Res 2002; 74: 237- 244.
6. Wong WW. A hypothesis on the pathogenesis of pterygium. Ann Ophthalmol 1978; 10: 303-308.
7. Hill JC, Maske R. Pathogenesis of pterygium. Eye 1989; 3: 218-226.
8. Coroneo MT, Di Girolamo N, Wakefield D. The pathogenesis of pterygia. Curr Opin Ophthalmol 1999; 10: 282-288.
9. Chowers I, Peer J Ehud Zamir E, et al. Proliferative activity and p53 expression in primary and recurrent pterygia. Ophthalmology 2001; 108: 985-988.
10. Marcovich AL, Morad Y, Sandbank J, et al. Angiogenesis in pterygium: morphometric and immunohistochemical study. Curr Eye Res 2002; 25: 17-22.
11. Lee DH, Cho HJ, Kim JT, et al. Expression of vascular endothelial growth factor and inducible nitric oxide synthase in pterygia. Cornea 2001; 20: 738-742.
12. Gebhardt M, Mentlein R, Schaudig U, et al. Differential expression of vascular endothelial growth factor implies limbal origin of pterygia. Ophthalmology 2005; 112: 1023-1030.
13. Jin J, Guan M, Sima J, et al. Decreased pigment epithelium derived factor and increased vascular endothelial growth factor levels in pterygia. Cornea 2003; 22: 473-477.
14. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335-2342.
15. Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci 2000; 41: 2514-2522.
16. Miller JW, Adamis AP, Aiello LP. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab Rev 1997; 13: 37-50.
17. Kim KJ, Li B, Winer J, et al. Inhibition of vascular-endothelial-growth-factor-induced angiogenesis suppresses tumor growth in vivo. Nature 1993; 362: 841-844.
18. Michels S, Rosenfeld PJ, Puliafito CA, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: twelve-week results of an uncontrolled open-label clinical study. Ophthalmology 2005; 112: 1035-1047.
19. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Laser Imaging 2005; 36: 331-335.
20. Avery RL, Pieramici DJ, Rabena MD. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 2006; 113: 363- 372.
21. Hirst LW. The treatment of pterygium. Surv Ophthalmol 2003; 48:146-180.
22. Sanchez-Thorin JC, Rocha G, Yelin JB. Metaanalysis on the recurrence rates after
bare sclera resection with and without mitomycin C use and conjunctival autograft placement in surgery for primary pterygium. Br J Ophthalmol 1998; 82: 661-665.
23. Tan DTH, Chee SP, Dear KBG, Lim ASM. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol 1997; 115: 1235-1240.
24. Manzano R, Peyman G, Khan P, et al. Inhibition of experimental corneal neovascularization by bevacizumab (Avastin). Br J Ophthalmol 2007; 91: 804-807.
Acknowledgment
The authors would like to thank Johann Michael Reyes, MD; Romeo Dela Cruz, MD; Richard Nepomuceno, MD; and Ma. Grace Rosales for their invaluable assistance.

