Safety of Intracameral Moxifloxacin/Dexamethasone Fixed-Dose Formulation on the Corneal Endothelium in a Rabbit Model
Reginald Robert Tan, MD, Joseph Anthony Tumbocon, MD, Ruben Lim Bon Siong, MD, Jay Marianito Vicencio, MD
There has been an increasing trend of giving antibiotics intracamerally after surgery and numerous studies have recommended its use. Results of the multicenter European Society of Cataract & Refractive Surgery (ESCRS) Guidelines for Prevention and Treatment of Endophthalmitis Following Cataract Surgery concluded that even after basic local antisepsis techniques, intracameral injection of cefuroxime at the end of surgery further decreased the incidence of endophthalmitis rates from 0.3 to 1.2 percent to rates of only 0.014 to 0.08 percent.1 However, cefuroxime has limited coverage to common pathogens causing endophthalmitis and the cost of this drug is prohibitive. In more recent years, off-label intracameral use of the preservative-free moxifloxacin ophthalmic solution (Vigamox, Alcon Laboratories, Fort Worth, TX) was established to be safe and effective when used at the conclusion of cataract surgery.2
In contrast, the role of intracameral steroids after intraocular surgery have not been clearly established. Nonetheless, a recent study showed that intracameral injection of 0.4 mg dexamethasone after intraocular surgery was able to decrease inflammation and improve surgical outcomes without having any adverse effects, like intraocular pressure (IOP) elevation in both glaucomatous and non-glaucomatous eyes.3
More recently, a 0.5% moxifloxacin/0.1% dexamethasone fixed-dose combination topical solution (Vigadexa, Alcon Laboratories, Fort Worth, TX) has become available. It is also preservative-free, has a potential use for intracameral administration, and has an added anti-inflammatory activity as compared to preservative-free moxifloxacin. However, there were no studies on the safety of this drug when injected intracamerally.
The purpose of this study was to determine the safety of intracamerally injected preservative-free fixed-combination 0.5% moxifloxacin/0.1% dexamethasone (IPFMDex) on the corneal endothelium in rabbit models and compare it to intracamerally injected preservative-free 0.5% moxifloxacin (IPFM) as control.
More specifically, we compared the corneal endothelial cell count, corneal thickness, corneal clarity, and anterior chamber inflammation at Day 1 and Day 7 postinjection with baseline (Day 0). We also compared the effect on corneal endothelial cell count, corneal thickness, corneal clarity, anterior chamber inflammation, and endothelial cell damage between the IPFMDex and IPFM groups on Days 1 and 7 postinjection.
METHODOLOGY
This experimental study was approved by the Institutional Review Board (IRB) and the Institutional Animal Care and Use Committee (IACUC) of St. Luke’s Medical Center. All practices in this study were done in accordance to the Association for Research in Vision and Ophthalmology (ARVO) guidelines for use of animals in ophthalmic and vision research.
Six male and four female albino rabbits, 4 to 13 months old, were acquired from the St Luke’s Medical Center Research and Biotechnology Division (RBD) animal facility. The rabbit’s right eyes were assigned as the IPFM group (control) and the left eyes the IPFMDex group (experimental).
Procedure
All ten rabbits were systemically anesthesized using ketamine hydrochloride (Ketamax, Rotexmedica GmbH, Trittau, Germany) 50 mg/kg injected into the rear flank muscle. The rabbit eyes were examined under slit-lamp biomicroscopy for baseline corneal clarity (0 = absense of corneal haze, 1 = presence of corneal haze) and presence of ocular inflammation in the form of anterior chamber (AC) cells (0 = no cells, 1 = presence of cells).
All eyes underwent specular microscopy and pachymetry (SP-9000, Konan Medical Inc., Hyogo, Japan) operated by a single senior technician. Corneal endothelial cell count (ECC) was measured using center to center method. Three images were taken and the image with the best quality was utilized. At least 120 well-defined endothelial cells were marked for analysis. Central corneal thickness (CT) was measured three times using the same instrument and the average value was obtained. Baseline values were recorded as Day 0.
Topical proparacaine 0.5% (Alcaine, Alcon Laboratories, Fort Worth, TX) was instilled into the conjunctival sac before the intracameral injections. After aspiration of 0.1 mL of aqueous humor using a g30 one half-inch needle in a 1 mL syringe, 0.1 mL of 0.5% moxifloxacin (Vigamox, Alcon Laboratories, Fort Worth, TX) was injected into the anterior chamber of the right eye (IPFM group) and 0.1 mL of fixed-combination 0.5% moxifloxacin/0.1% dexamethasone (Vigadexa, Alcon Laboratories, Fort Worth, TX) was injected into the anterior chamber of the left eye (IPFMDex group).
Twenty-four hours after injection, five rabbits were randomly chosen by fishbowl technique and all ten eyes were examined using biomicroscopy for AC reaction and corneal clarity. The eyes underwent specular microscopy and corneal pachymetry and data was recorded under Day 1. The rabbits were euthanized using overdose of ketamine, and the eyes enucleated.
The corneas were carefully harvested along with perilimbal sclera using blade and sharp scissors and the endothelium was exposed to trypan blue and alizarin red for vital staining. The corneal endothelium was covered with drops of 0.25% trypan blue (Sigma-Aldrich, St. Louis, MO) for 120 seconds; then rinsed twice with balanced salt solution (BSS). The excess saline was drained. Likewise, 0.2% alizarin red S (Sigma-Aldrich, St. Louis, MO) was added to cover the endothelium for 90 seconds; then rinsed twice with BSS. Areas with blue and red dye staining represented damaged endothelium; trypan blue stained dead cell nuclei and alizarin red stained descemet membrane areas with dislodged endothelial cells. Otherwise, a normal healthy endothelial cell with an intact plasma membrane would not take up the dyes. Each sample was placed on a tissue culture well with balanced salt solution (BSS), endothelial side up. Digital capture of the whole span of the endothelium using an 8 megapixel camera through a microscope (Carl Zeiss M:SV11) was done with 16x magnification.
Following the protocol of Saad et al,4 quantitative analysis of the endothelial cell damage (ECD) was achieved by using the Adobe Photoshop 12.0 software program. By quantifying selected areas with dye uptake and dividing it by the quantified total graft area, the amount of ECD in percentage was calculated. To be more specific, after using the Magnification tool and the Quick Selection tool to select the borders of the corneal graft, the Image/Histogram function revealed the selected total graft area count in pixels. Subsequently, areas with endothelial damage (areas with trypan blue and alizarin red staining) were selected using the Magic Wand tool, then quantified using the Image/Histogram function.
After 7 days, the 10 remaining rabbit eyes were subjected to slit-lamp biomicroscopy, pachymetry, and specular microscopy. They were euthanized using the same method and the eyes enucleated for corneal vital staining and subsequent quantitative analysis.
Primary outcome measures in this study included corneal endothelial cell count (ECC) and endothelial cell damage (ECD). Secondary outcome measures were corneal thickness (CT), corneal clarity, and AC inflammation.
Statistical Analysis
The ten rabbits were divided into two subgroups for analysis: Subgroup A (IPFM-A, IPFMDex-A) were euthanized at Day 1 and subgroup B (IPFM-B, IPFMDex-B) Day 7.
ECC and CT on Day 1 (group A) and Day 7 (group B) were compared to baseline values at Day 0 of each respective group using paired sample t-test. The ECC, CT, and ECD of the IPFMDex group were compared with the IPFM group at Day 0, Day 1, and Day 7 using an independent sample t-test. A p value less than 0.05 was considered statistically significant. Statistical analyses were carried out using Excel version 14.1 (Microsoft Corporation, Seattle, WA, USA).
RESULTS
Endothelial cell count
The mean endothelial cell count for the different subgroups are shown in Table 1. There were no significant differences between Day 0 and 1 for IPFM-A, between Day 0 and 7 for IPFM-B, between Day 0 and 1 for IPFMDex-A, and between Day 0 and 7 for IPFMDex-B (Table 1).
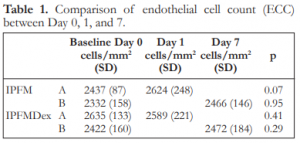
Table 1. Comparison of endothelial cell count (ECC) between Day 0, 1, and 7.
IPFM – intracameral moxifloxacin, IPFMDex – intracameral moxifloxacin/dexamethasone, SD – standard deviation
There were also no significant differences in the endothelial cell count between the IPFM and IPFMDex groups at Day 0, 1, and 7 (Table 2).
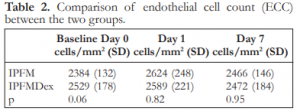
Table 2. Comparison of endothelial cell count (ECC) between the two groups.
IPFM – intracameral moxifloxacin, IPFMDex – intracameral moxifloxacin/dexamethasone, SD – standard deviation
Cell Viability
The mean endothelial cell damage for the IPFM was 2.2% and 3.2% at Day 1 and 7, respectively; for IPFMDex 2.2% and 3.7% at Day 1 and 7, respectively.There were no significant differences in endothelial cell damage between the two groups (Table 3).

Table 3. Comparison of endothelial cell damage (ECD) between the two groups.
IPFM – intracameral moxifloxacin, IPFMDex – intracameral moxifloxacin/dexamethasone, SD – standard deviation
Central corneal thickness
The mean corneal thickness for the subgroups are shown in Table 4. There were no significant differences between Day 0 and 1 for IPFM-A, between Day 0 and 7 for IPFM-B, between Day 0 and 1 for IPFMDex-A, and between Day 0 and 7 for IPFMDex-B (Table 4).
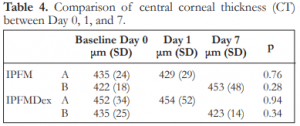
Table 4. Comparison of central corneal thickness (CT) between Day 0, 1, and 7.
IPFM – intracameral moxifloxacin, IPFMDex – intracameral moxifloxacin/dexamethasone, SD – standard deviation
There were no significant differences in the corneal thickness between the IPFM and the IPFMDex groups at Day 0, 1, and 7 (Table 5).

Table 5. Comparison of central corneal thickness (CT) between the two groups
IPFM – intracameral moxifloxacin, IPFMDex – intracameral moxifloxacin/dexamethasone, SD – standard deviation
Corneal clarity and AC inflammation
At all biomicroscopy performed on Day 0, 1, and 7, the corneas were clear and showed no signs of AC inflammation in both the IPFM and IPFMDex groups.
Adverse events
Two rabbit corneas on Day 7 of the IPFM group sustained significant descemet membrane (DM) tears during the harvesting of the cornea. These corneas were not excluded from the study and calculation of endothelial cell damage was computed for by dividing the number of pixels making up the dye-stained areas by the number of pixels making up the remaining graft area without DM tear.
DISCUSSION
This study showed that intracameral preservative-free 0.5% moxifloxacin/0.1% dexamethasone was as safe as preservative-free 0.5% moxifloxacin in rabbit eyes. This supported previous findings of the latter drug.2,5-7 Espiritu et al.5 demonstrated that moxifloxacin was nontoxic when given intracamerally at the conclusion of cataract surgery. In fact, intracameral moxifloxacin was as safe as giving BSS.6 Further evaluation using scanning and transmission electron microscopy one day after intracameral moxifloxacin injection showed preserved endothelial cell appearance, hexagonality, and cell architecture.7
In our study, the addition of dexamethasone to moxifloxacin as a fixed-dose combination formula did not increase its toxicity to the cornea when injected intracamerally in rabbit eyes. This confirmed the findings of more recent studies on dexamethasone, suggesting its safety when given intracamerally.3,8 Indeed, this drug was not harmful to the corneal endothelium when given intracamerally at the end of cataract surgery by phacoemulsification.8
To the author’s knowledge, this was the first study that investigated the safety of giving intracameral injection of preservative-free 0.5% moxifloxacin/0.1% dexamethasone fixed-dose formulation on the corneal endothelium.
A limitation of the current study was that it only tested for the safety of moxifloxacin/dexamethasone on the corneal endothelium. Other parameters, such as effectivity in preventing infection or controlling inflammation, were not tested. Future studies testing the efficacy of moxifloxacin/dexamethasone in controlling inflammation when given intracamerally in animal models or in humans are warranted.
In conclusion, intracameral injection of preservative-free moxifloxacin/dexamethasone fixed-dose formulation was safe on the rabbit corneal endothelium and was no different from preservative-free moxifloxacin.
REFERENCES
1. Barry P, Cordovés L, Gardner S. ESCRS guidelines for prevention and treatment of endophthalmitis following cataract surgery: data, dilemmas, and conclusions. Paper presented at: the European Society of Cataract and Refractive Surgeons; 2013; Dublin, Ireland.
2. Matsuura K, Miyoshi T, Suto C, et al. Efficacy and safety of prophylactic intracameral moxifloxacin injection in Japan. J Cataract Refract Surg 2013;39:1702-1706.
3. Chang DT, Herceg MC, Bilonick RA, et al. Intracameral dexamethasone reduces inflammation on the first postoperative day after cataract surgery in eyes with and without glaucoma. Clin Ophthalmol 2009;3:345-355.
4. Saad HA, Terry MA, Shamie N, et al. An easy and inexpensive method for quantitative analysis of endothelial damage by using vital dye staining and Adobe Photoshop software. Cornea 2008;27:818-824.
5. Espiritu CRG, Caparas VL, Bolinao JG. Safety of prophylactic intracameral moxifloxacin 0.5% ophthalmic solution in cataract surgery patients. J Cataract Refract Surg 2007;33:63-68.
6. Lane SS, Osher RH, Masket S, et al. Evaluation of the safety of prophylactic intracameral moxifloxacin in cataract surgery. J Cataract Refract Surg 2008;34:1451-1459.
7. Kim SY, Park YH, Lee YC. Comparison of the effect of intracameral moxifloxacin, levofloxacin, and cefazolin on rabbit corneal endothelial cells. Clin Exp Ophthalmol 2008;36:367-370.
8. Jamil AZ, Ahmed A, Mirza KA. Effect of intracameral use of dexamethasone on corneal endothelial cells. J Coll Physicians Surg Pak 2014;24:245-248.

