Photodynamic therapy for neovascular age-related macular degeneration
Richard P.L. Wormald, MA, MB, BCh,MSc, FRCS,FRCOphth, Jennifer R Evans, PhD, Katherine S Henshaw, Liam L Smeeth, MD
BACKGROUND
Age-related macular degeneration (ARMD) is a disease affecting the macula, the central area of the retina. The disease is defined as degeneration of the macula in older people (aged over 50) with no other apparent cause for the degeneration. There are several signs in the retina that are associated with increasing age and increased risk of developing ARMD.
These signs, known as age-related maculopathy, include the presence of drusen (yellow spots beneath the retina) and pigmentary disturbance. In general, age-related maculopathy is not associated with visual loss. Some people with age-related maculopathy will go on to develop ARMD. There are 2 main types of ARMD. In geographic atrophy (dry) ARMD, the retinal pigment epithelium is lost completely in localized areas. In neovascular (wet) ARMD, subretinal neovascular membranes (new blood vessels) develop beneath the retina. These are associated with scarring of the retina that affects vision. The new vessels can leak causing hemorrhage that leads to larger scars or macular edema and significant loss of vision. This review was concerned with treatment for neovascular age-related macular degeneration. Subretinal neovascular membranes are defined as classic or occult according to their appearance on fluorescein angiography, in which fluorescent dye is injected intravenously and photographed as it passes through the blood vessels of the eye. Classic membranes are clearly delineated and leak fluorescein uniformly. Occult membranes are often hidden or their extent is hard to delineate, and fluorescein leakage is patchy. It is thought that these 2 angiographic patterns reflect the different extent to which the vessels have penetrated the retinal pigment epithelium, occult vessels lying underneath the retinal pigment epithelium. Some lesions may have both classic and occult components. Trials have shown that early laser photocoagulation of classic extrafoveal membranes (those not directly underneath the fovea at the center of the macula) could delay the loss of vision in a small number of patients.1 However, most patients present with subfoveal membranes, and while photocoagulation can limit the extent of the subsequent visual loss, it causes immediate loss of central vision due to the concurrent destruction of the overlying retina. Photodynamic therapy (PDT), originally used in the treatment of cancer, has been investigated as a way to treat the neovascular membranes without affecting the retina. Photoreactive chemicals are injected into the patient and irradiated with light as they pass through the neovascular membranes. This light is strong enough to activate the chemicals, causing them to emit free radicals that destroy the blood vessels, but is not strong enough to cause damage to the overlying retina.
OBJECTIVE
The aim of this review was to examine the effects of photodynamic therapy in the treatment of neovascular ARMD.
CRITERIA FOR CONSIDERING STUDIES FOR THIS REVIEW
Types of studies
We included randomized controlled trials.
Types of participants
We included trials in which participants were people with neovascular ARMD as defined by the study
investigators.
Types of interventions
We included any study in which photodynamic therapy
was compared to another treatment, placebo, or no
treatment.
Types of outcome measures
The primary outcome for this review was prevention of visual loss. Any well-defined outcome based on visual acuity was used depending on the way in which authors presented trial data. Other validated measures of visual loss, such as contrast sensitivity, were used where available. The secondary outcomes for this review were:
• new vessel growth;
• quality of life measures – any validated measurement scale that aims to measure the impact of visual function loss on quality of life of participants;
• any adverse outcomes as reported in trials.
SEARCH STRATEGY
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Group Trials Register) on The Cochrane Library, Medline, and EMBASE. We used the following strategy to search CENTRAL Issue 1, 2005:
#1 MACULAR DEGENERATION
#2 RETINAL DEGENERATION
#3 NEOVASCULARIZATION PATHOLOGIC
#4 (macula* or retina* or choroid*)
#5 (degenerat* or neovascular*)
#6 (#4 and #5)
#7 maculopath*
#8 (#1 or #2 or #3 or #6 or #7)
#9 PHOTOCHEMOTHERAPY
#10 PHOTOSENSITIZING AGENTS
#11(photosensit* or photodynamic* or pdt or verteporfin or visudyne)
#12 (#9 or #10 or #11)
#13 (#8 and #12)
We used the following strategy combined with the Cochrane highly sensitive search strategy2 to search MEDLINE on SilverPlatter to January 2005.
#1 explode “Macular-Degeneration”/all SUBHEADINGS in MIME,MJME
#2 explode “Retinal-Degeneration” / all SUBHEADINGS in MIME,MJME
#3 explode “Choroidal-Neovascularization” / all SUBHEADINGS in MIME,MJME
#4 ( ((macul* or retina* or choroid*)near (degener* or neovasc*)) in TI )or( ((macul* or retina* or choroid*)near (degener* or neovasc*)) in AB )
#5 maculopath* in TI
#6 maculopath* in AB
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 explode “Photochemotherapy-” / all SUBHEADINGS in MIME,MJME
#9 explode “Photosensitizing-Agents” / all SUBHEADINGS in MIME,MJME
#10 ( ((photosensiti* near agent*)or porphyrin* or benzoporphyrin*) in AB )or( ((photosensiti* near agent*)or porphyrin* or benzoporphyrin*) in NM ) or ( ((photosensiti* near agent*)or porphyrin* or
benzoporphyrin*) in TI )
#11 ( (photodynamic* or PDT) in AB ) or ( (photodynamic* or PDT) in TI )
#12 ( (verteporfin or visudyne) in AB )or ( (verteporfin or visudyne) in TI )
#13 #8 or #9 or #10 or #11 or #12
#14 #7 and #13
We used the following strategy to search EMBASE on Ovid to January 2005.
1. exp Retina Macula Age Related Degeneration/
2. exp Retina Degeneration/
3. exp “Neovascularization (Pathology)”/
4. exp Subretinal Neovascularization/
5. ((macul$ or retina$ or choroid$) adj5 (degener$ or neovasc$)).ab,ti.
6. maculopath$.ab,ti.
7. 1 or 2 or 3 or 4 or 5 or 6
8. exp Photodynamic Therapy/
9. exp Photosensitizing Agent/
10. (photodynamic$ or PDT).ab,ti.
11. (photosensit$ adj3 agent$).ab,ti.
12. (verteporfin or visudyne).ab,tn,ti.
13. 8 or 9 or 10 or 11 or 12
14. 7 and 13
To identify randomized controlled trials, we combined this search with the following strategy:
#1 Randomized Controlled Trial/
#2 exp Randomization/
#3 Double Blind Procedure/
#4 Single Blind Procedure/
#5 random$.ab,ti.
#6 #1 or #2 or #3 or #4 or #5
#7 (animal or animal experiment).sh.
#8 human.sh.
#9 #7 and #8
#10 #7 not #9
#11 #6 not #10
#12 Clinical Trial/
#13 (clin$ adj3 trial$).ab,ti.
#14 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).ab,ti.
#15 exp PLACEBO/
#16 placebo$.ab,ti.
#17 random$.ab,ti.
#18 experimental design/
#19 Crossover Procedure/
#20 exp Control Group/
#21 exp LATIN SQUARE DESIGN/
#22 #12 or #13 or #14 or #15 or #16 or #17 or #18 or#19
or #20 or #21
#23 #22 not #10
#24 #23 not #11
#25 exp Comparative Study/
#26 exp Evaluation/
#27 exp Prospective Study/
#28 (control$ or prospectiv$ or volunteer$).ab,ti.
#29 #25 or #26 or #27 or #28
#30 #29 not #10
#31 #30 not (#11 or #23)
#32 #11 or #24 or #31
Manual searches
We used the Science Citation Index to search for reports that cited relevant study reports. We contacted experts in the field for information about further trials and we searched the reference lists of relevant studies for further trial reports.
METHODS OF THE REVIEW
Selection of trials
Two authors independently scanned the titles and abstracts resulting from the electronic searches. We obtained full copies of all potentially or definitely relevant articles. Two review authors assessed the full copies according to the “Criteria for considering studies for this review.” Only articles meeting these criteria were assessed for quality. Assessment of methodological quality Two authors independently assessed study quality
according to methods set out in Section 6 of the Cochrane Handbook for Systematic Reviews of Interventions.3
The authors were not masked to any trial details during the assessment. Four parameters of quality were considered: allocation concealment and method of allocation to treatment, masking of providers and recipients of care, masking of outcome assessment, and completeness of follow up. Each parameter of trial quality was graded: A (adequate); B (unclear); C (inadequate). Disagreement between the review authors on assessments was resolved by discussion. We contacted the trial authors for clarification on any parameter graded B and we excluded any trial scoring on allocation concealment.
Data collection
Two authors independently extracted data using a form developed by the Cochrane Eyes and Vision Group (available from the editorial base). We resolved discrepancies by discussion. Two review authors independently entered data into RevMan 4.2 (The Cochrane Collaboration, Oxford, United Kingdom) and we checked any inconsistencies between the two against the study report.
Data synthesis
Our original data analysis plan was to summarize data from studies collecting similar outcome measures with similar follow-up times using the Peto method, after testing for heterogeneity between trial results using a standard chi square test. The main outcome analyzed, loss of three or more lines of visual acuity at 12 and 24 months follow up, occurred relatively frequently in the trial cohort. The odds ratio, therefore, does not approximate the relative risk. We present relative risks in this review. We planned to conduct sensitivity analyses to determine the effect of excluding studies given a grade of C (inadequate) on any parameter of quality but to date this has not been necessary.
DESCRIPTION OF STUDIES
Finding the trials
The original electronic searches identified 76 reports. We found one randomized controlled trial (TAP 1999).4 Since the searches were updated in February 2001, May 2002, and January 2003, one further study has been identified and included in the review (VIP 2001) (Table 1).5 A further search update was conducted in January 2005. A total of 284 new reports were found. No reports of new trials were found though there were a number of new reports from existing trials including new outcomes on contrast sensitivity,6 central-visual-field function,7 and subretinal neovascular morphology.8 In addition we found one systematic review,9 a metaanalysis of safety results in
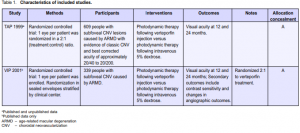
TAP and VIP10 and a cost-utility analysis.11 A report on severe visual-acuity decrease in TAP and VIP12 was also considered relevant. An outcome study reporting visual function and related quality of life was found.13 A number of papers from the TAP and VIP studies were found including guidelines for evaluation of fluorescein angiographic findings and treatment,14 determinants of outcome according to lesion size, visual acuity and lesion composition,15 baseline lesion composition’s impact on vision outcome,16 and natural history of minimally classic lesions.17 We found no reports from ongoing trials (Table 2) but one traditional review of PDT18 mentions trials on other agents, such as etiopurpurin (Purlytin) and motexafin lutetium (Optrin) undergoing phase III and phase II trials respectively.
Summary of the characteristics of included studies Table 1 shows the summary and details of the included studies. TAP 1999 was a multicenter study that investigated the safety and effectiveness of verteporfin (Visudyne; CIBA Vision Corp, USA). It was conducted in 22 ophthalmology practices in Europe and North America. Participants were people with subfoveal choroidal neovascularization (CNV) caused by ARMD. The majority of participants were white (98%) with a mean age of 75 years. TAP 1999 was originally devised as 2 concurrent trials in order to comply with regulatory agency requirements. The study protocols were identical. Ten of the clinical centers were assigned to study. A and 12 to study B. As the results of the trials were similar and the investigators analyzed and presented the data as one trial we have also assessed them as one trial. The VIP 2001 study was very similar to the TAP 1999 study. It was conducted in 28 practices, most of whom had also participated in TAP 1999. As for TAP 1999, most of participants were white (98%) with a mean age of 75 years. In both trials verteporfin (6 mg/m2 body surface area) was compared to placebo (5% dextrose in water) administered via intravenous infusion of 30 ml over 10 minutes. This was followed after 15 minutes by application of 83 seconds of laser light at 689 nm delivered 50 joules/cm2 at an intensity of 600 mW/cm2 using a spot size with a diameter 1000 microns larger than the greatest linear dimension of the CNV lesion. Participants in TAP 1999 were reviewed every 3 months when visual acuity was measured and repeat fluorescein angiography performed. If the trial surgeon judged a recurrence of the membrane to be present or a persistence of the previous lesion, then repeat treatment was undertaken. In the phase one and two studies, it was concluded that up to 5 treatments were necessary to stabilize the situation.19, 20 In the first year, a mean of 3.4 treatments were delivered to the treatment group and 3.7 to the control group. In the second year a mean of 2.2 treatments were delivered to the treatment group and 2.8 to the control group. Visual acuity was measured in VIP 2001 at 12 and 24 months. The report of the study did not indicate the mean number of treatments delivered for all participants. However, in the subgroup with occult CNV (76% of all participants) 3.1 treatments were given in the treatment group and 3.5 in the control group. In the second year,
1.8 and 2.4 treatments were given in the verteporfin and control groups, respectively. Methodological quality of included studies Both TAP 1999 and VIP 2001 were high-quality studies with a very similar study design. Allocation of treatment group was by opaque serially numbered sealed envelopes and stratified by clinical center. The baseline characteristics of the participants by treatment group were published. The groups were well balanced with respect to a variety of demographic and clinical variables. Only 1 eye per person was treated. Reasonable attempts were made to mask the ophthalmologist, participant, vision examiner, and Photograph Reading Center personnel to the treatment assigned. As verteporfin and placebo are of different colors (green versus colorless), the solutions and the intravenous tubing were covered with foil. The fundus appearance did not change during treatment to indicate whether verteporfin or placebo had been infused. There was no other physical evidence of treatment as verteporfin dye is excreted in the feces and does not cause any color change; neither does it alter the color of the skin or urine. It was, therefore, unlikely that participants were aware of their treatment status. In TAP 1999, the study investigators reported 2 cases where the participants who were unmasked and 4 cases where the ophthalmologists who were unmasked noted a green solution. Rates of follow-up were high in both studies. In TAP 1999, 94% were seen at 12 months and 87% at 24 months. Follow-up was similar between the 2 treatment groups. The analysis was intention-to-treat, and subgroup analyses were planned a priori (Bressler N, personal communication). In VIP 2001, 93% were seen at 12 months and 86% at 24 months. All participants were included in the analyses and missing values were inputted using the method of last observation carried forward.
RESULTS
The realistic aim of photodynamic therapy is to slow down the progression of ARMD, not to produce normal vision. Outcomes were, therefore, expressed as risks of a
poor outcome rather than as improvements in vision. All results were based on the comparison of patients randomized to receive verteporfin with those randomized to receive placebo (control).
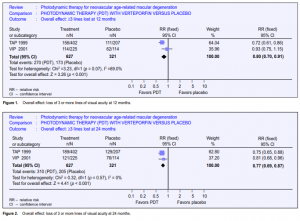
Overall analysis
Loss of 3 or more lines of visual acuity
A total of 948 participants from TAP 1999 and VIP 2001 were included in the metaanalysis. At 12 months, the pooled relative risk (RR) of losing 3 or more lines of visual acuity was 0.80 (Figure 1) and the relative risk reduction (RRR) was, therefore, 0.20 (95% CI 0.09 to 0.30). This analysis was done using a fixed-effects model. A randomeffects model gave a nonsignificant result, largely because it placed more weight on the VIP study (pooled RR 0.82; 95% CI 0.64 to 1.04). At 24 months, the pooled RR was 0.77 (Figure 2) and the RRR was, therefore, 0.23 (95% CI 0.13 to 0.31). The random-effects model yielded a similar result.
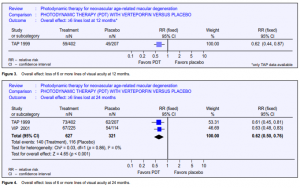
Loss of 6 or more lines of visual acuity
At 12 months, the RR of losing 6 or more lines of visual acuity was 0.62 (Figure 3) (TAP 1999 only, data not reported for VIP 2001). The RRR was, therefore, 0.38 (95% CI 0.13 to 0.56). At 24 months, the pooled RR was 0.62 (Figure 4). The RRR was 0.38 (95% CI 0.24 to 0.50).
Mean number of lines lost
In TAP 1999, the mean number of lines of vision lost at 12 months was 2.2 in the intervention group and 3.5 in the control group. The difference was 1.3, with fewer lines lost in the intervention group. The p value for the difference in the mean number of lines lost was reported as p < 0.001 (Wilcoxon rank sum test). At 24 months, the mean number of lines of vision lost was 2.7 in the intervention group and 3.9 in the control group, a difference of 1.2 lines (p < 0.001). The standard deviations for the mean numbers of lines lost were not reported and, therefore, the confidence intervals could not be calculated. Data on mean number of lines lost for the whole VIP 2001 study group were not reported.
Subgroup analyses
Subgroup data were available only for the outcome “loss of 3 or more lines of visual acuity” in TAP 1999 but for both outcomes (loss of 3 lines and loss of 6 lines) in VIP 2001.
Evidence of occult choroidal neovascularization
In TAP 1999, the RR of losing 3 or more lines of visual acuity at 12 months was 0.90 if CNV was present (95% CI 0.73 to 1.11) and 0.34 if occult CNV was absent (95% CI
0.22 to 0.51). At 24 months, the relative risks were 0.88 (95% CI 0.74 to 1.04) and 0.42 (95% CI 0.30 to 0.60) respectively. The test for effect modification between these 2 subgroups was significant. Neither the 95% nor the 99% confidence intervals for these 2 subgroups overlap.
Lesion area composed of classic choroidal neovascularization
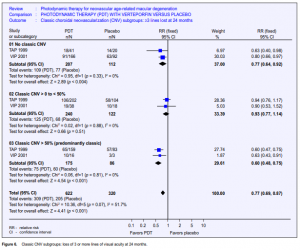
In TAP 1999, the proportion of the lesion composed of classic CNV was estimated as 0%; greater than 0% but less than 50%; greater than 50%. The proportion was unknown in 4 participants (3 in the treatment group and 1 in the control group). The subgroup analyses were, therefore, based on a total of 399 eyes. In VIP 2001, the majority of the participants (76%) had “occult with no classic CNV.” An additional 56 eyes had some classic CNV (less than 50% but greater than 0% as above). Only 19 eyes had predominantly classic CNV. The pooled RR for losing 3 or more lines of visual acuity at 12 months for the group with 0% CNV was 0.84 (95% CI 0.68 to 1.04). Results for 3 or more lines lost at 12 months were not reported for the other two subgroups in the VIP 2001 study. In TAP 1999, the RR for losing 3 or more lines of visual acuity at 12 months in people with more than 0% but less than 50% CNV was 0.99 and 0.54 for greater than 50% CNV (Figure 5). At 24 months, the pooled RR for losing 3 or more lines of visual acuity were 0.77, 0.93, and 0.60 (95% CI 0.48 to 0.75) respectively (Figure 6). These results suggested there was a reduction in the risk of loss of vision when classic CNV was absent or when greater than 50% of the lesion was composed of classic CNV. There was very little reduction in risk when between 0% and 50% of the lesion was comprised of classic CNV. However, the test for effect modification among these 3 subgroups was not statistically significant (p = 0.066).
Number needed to treat
We calculated the numbers needed to treat (NNTs) to prevent 1 person from losing 3 or more lines and, where possible, 1 person from losing 6 or more lines of vision. These NNTs were derived from the study population, that is, people with subfoveal CNV and a baseline visual acuity of between 20/40 and 20/200 with approximately 5 treatments over 2 years. The NNT to prevent one person from losing 3 or more lines of vision at 24 months was 7.1 (95% CI 4.8 to 12.5). The NNT to prevent 1 person from losing 6 or more lines of vision at 24 months was 7.1 (95% CI 5.0 to 12.5).
Other primary outcomes
Contrast sensitivity
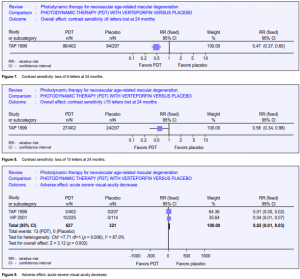
This outcome from the TAP trial was reported by Rubin 2002.6 It was measured in participants at baseline and at three-month intervals after refraction and measurement of best-corrected visual acuity. Contrast sensitivity was measured using the Pelli Robson chart (no. 7002251 Clement Clarke, Columbus, Ohio). The measurements were made using a standard protocol and illumination and outcomes were categorized in terms of more than 6 or more than 15 letters lost since baseline. A higher proportion of those treated with placebo lost both more than 6 and 15 letters of contrast sensitivity at 12 and 24 months. The RR of losing 6 lines of contrast sensitivity by 24 months was 0.47 in the PDT group compared to placebo (Figure 7). For 15 letters, the RR was 0.58 (Figure 8).
Central-visual-field function
This was reported by Schmidt-Erfurth7 for 46 participants of the TAP trial based in Germany. Participants in this center had various additional investigations, including Scanning Laser Ophthalmoscopic (SLO) perimetry of the

macula to measure the size of the central scotoma in treated and placebo groups. It was given as mean area in mm.2 The mean area of the absolute scotoma increased in both groups but significantly more in the placebo arm (2.5 mm2 baseline to 7.3 mm2 at 24 months in the treated group compared to 2.7 mm2 at baseline to 31.5 mm2 at 24 months in the placebo group). Similar findings were reported for differences in the increase in the size of the relative scotoma between groups. These differences were statistically significant at the level of p < 0.001 though neither standard errors of these means nor 95% confidence intervals were provided.
Secondary outcomes
Neovascular-membrane morphology
Schmidt-Erfurth’s group also reported on the outcome of Confocal Indocyanine Green (ICG) angiography in the subgroup of the TAP trial participants in Germany.8 In this case, outcomes for 60 participants were reported. It is not clear why there was a discrepancy between the 60 participants in this analysis and the 46 who underwent measurement of central scotoma as described above. Presumably 14 patients did not have SLO perimetry but did have ICG angiography. This paper reported outcomes in terms of the mean size of the neovascular membrane in mm2. Forty eyes received PDT and 20 received placebo. Baseline mean areas of ICG leakage were 3.9 mm2 for the PDT group and 2.8 mm2 for the placebo eyes. This reduced to 3.0 mm2 in the treated group at 24 months compared to a growth to 9.6 mm2 in placebo eyes. This difference was reported as highly significant (p = 0.008), but no standard errors or confidence limits were provided apart from graphically represented error bars that were not specified in the legend.
Quality of life
Evidence of efficacy as described above has still not been substantiated by any quality of life outcomes reported from the TAP or VIP trials.
Adverse effects
More information on this has become available for this update. In particular, the risk of severe and profound
visual loss has been better estimated. This has been provided by two reports from the TAP12 and VIP10
investigators and a large phase 4, open-label study that reported on the outcomes of verteporfin PDT in 4,435 patients called the VAM study.21
Arnold 200412 focused on the occurrence of acute severe visual-acuity decrease (ASVAD). This was defined
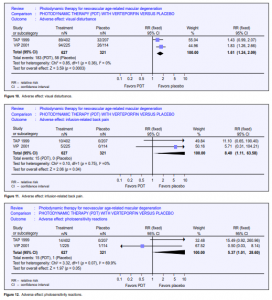
as at least a 20-letter loss (equivalent to 4 lines) within 7 days after treatment. Even though this paper reported this outcome from 2 RCTs, they described the study as an observational case series and a fairly detailed account was given of 15 events in 14 eyes. One of these was later judged as unlikely to be due to PDT. All but 2 events occurred shortly after the first treatment and only in the treated arm. Three of these events occurred in the TAP trial and 10 in the VIP. All 13 events occurred within 3 days of treatment. The absolute risk difference for both studies is 0.02 (Figure 9). The number needed to harm (NNH)
is estimated at 50 (range 30 to 100). That is, 1 eye will experience ASVAD in 50 treatments. Azab 200410 provided these data in the context of all other adverse events reported for the 2 trials. This report was described as a metaanalysis although data were only combined for the 2 trials for systemic side effects. The
authors found that only visual disturbances including ASVAD, injection-site reactions, photosensitivity reactions, and infusion-related back pain occurred with greater frequency in the treated participants.
The VAM study21 reported the outcomes from a larger number of patients recruited from 222 centers in North
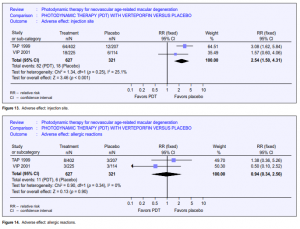
America (10 times the number in TAP) between September 1999 and June 2000 when verteporfin became commercially available. Maximum follow-up was 9 months. About half the study population had 6 months follow-up. This study provided further information on the risk of adverse events outside an RCT setting, but as this is an open-label study with no comparator group, relative risks or risk differences (and hence NNH) cannot be calculated. However, it was assumed that, as in TAP and VIP, no events would have occurred in an untreated arm, hence the risk became the same as the risk difference. Of the 4,435 enrolled, 115 (2.6%) reported abnormal or decreased vision, of whom 25 experienced ASVAD (0.6%) (NNH at 166). ASVAD was thought to be caused by bleeding under the retina after PDT. One series from Wilmer22 reported this outcome in 52 patients; unfortunately, the denominator (the overall number of persons and eyes receiving PDT) was not given. Vision loss can be profound in this group, and the data from TAP and VIP suggested it may be more likely to occur in people with better initial visual acuity. Visual disturbance (reports of “abnormal vision,” “decreased vision,” and visual-field defect) occurred in 1 in every 4 people who took part in the TAP 1999 and VIP 2001 studies. This is perhaps unsurprising as participants had neovascular ARMD. However, people treated with verteporfin were more likely to report visual disturbance (pooled relative risk 1.61) (Figure 10). Presumably, this visual disturbance must have been reasonably transient as visual outcomes at 12 and 24 months were better in the treatment group. 2.4% of people treated with verteporfin experienced infusion-related back pain (Figure 11) and 2.4% had photo-sensitivity reactions (Figure 12). Problems with the injection site occurred in 13.1% of people treated with verteporfin compared with 5.6% people in the control group (Figure 13). Few allergic reactions were seen and these were equally likely in the treatment and control groups (Figure 14).
Economic outcomes
No economic analyses have been reported from either TAP or VIP.
DISCUSSION
The absence to date of any effective treatment for neovascular ARMD (except for a few patients in whom
laser photocoagulation works) means that there will be intense interest in PDT for the many millions of sufferers of the disease worldwide. Unfortunately, PDT, like photocoagu-lation, can be effective only during the
proliferative stage of the disease while the neovascular process is active. It cannot have any effect once sight is
lost and the scarring process is complete. Therefore, like in so many other degenerative processes of the
neuroretina, nothing can be done to restore function once the damage is done. Most sufferers of the condition have established sight loss, and for them, the publicity surrounding the launch of verteporfin (Visudyne) would have raised false hopes. However, this review indicates that for people with active neovascular disease, PDT can prevent vision loss. This is corroborated by additional outcome measures such as contrast sensitivity, size of central scotoma, and neovascular-membrane dimensions. A key question is how long the effect of treatment will last and whether repeated treatments would be required in the longer term. This review indicates that treatment benefits last for at least 2 years. An open-label extension of the TAP 1999 study indicated that vision outcomes remained relatively stable for 24 to 48 months.23 There have been no further reports of longer outcomes. Another important issue is how many presenting patients will benefit from PDT. In addition to the problem of accessing specialist services in time, there is the question of the proportion of lesions that will actually be treatable. The evidence reported here clearly suggests that purely classic neovascular membranes do well. Subgroup analysis of the TAP 1999 study suggested that PDT is not effective when occult CNV is present. Occult vessels mean that the extent of the membrane cannot be clearly defined and so it is not surprising that treatment was found to be less effective because the laser cannot be aimed at the entire membrane. However, the VIP 2001 study recruited mostly patients with occult neovascularization and demonstrated treatment benefit at 12 and 24 months. Pooled analysis of the TAP 1999 and VIP 2001 studies in this review showed no statistically significant difference in treatment effects in subgroups defined by the presence or absence of classic CNV. This observation has been noted by other authors. For example, Meads 2004 cast serious doubt on the validity of the subgroup analyses.9 Subsequent reports of “exploratory” analyses (presumably not specified a priori) have been published from the TAP trials (Bressler 200216) and from the TAP and VIP trials (Blinder 200315), which found that only lesion size (the smaller lesions do better) and poorer presenting acuity (perhaps less vision to lose) were predictors of better outcome. One other report from TAP (Bressler 200417) examined the natural history of minimally classic lesions that had poorer outcomes in the TAP trial treated group. Of the 207 randomized to the placebo group, 98 had minimally classic lesions of which 39 progressed to become predominantly classic (21 of these within three months). The suggestion here is that it might be advisable to wait for minimally classic lesions to progress to predominantly classic so that the potential effectiveness of PDT might be greater. The authors imply that this need not necessarily be at the expense of allowing the lesion to become very large or indeed the vision to deteriorate. We are not told in the available reports the extent to which clinicians and the Photograph Reading Center personnel were able to agree on the subgroup classification of classic or occult lesions. It was likely that there was much variation in opinion on this. The necessary skill to report on fluorescein angiograms and recognize different lesion types is highly refined. Most experts assert that stereo images are required to be able to locate the position in depth of staining or fluorescein leaks. Stereophotography requires either a dedicated camera equipped to take simultaneous stereo images or a skilled photographer who takes sequential images slightly laterally
displaced from one another, providing a nonsimultaneous or pseudo-stereo image. However, the guidelines for reporting angiograms and data on interobserver agreement have now been published for the TAP and VIP
trials.14 A lot of detail is given on reporting guidelines but the information on agreement is somewhat brief though reported kappa values for the main subgroup criteria were good. This was based on a 10% subsample of graded photographs. Another independent study has reported on agreement within and between 16 different specialists in Germany24 for the same angiographic criteria as for TAP and VIP. Agreement was not quite as good for both intra- and interobserver as for the reporting center for the trials, but was acceptable nevertheless. The natural history of the growth of subretinal membranes varies from individual to individual. They may be aggressive and rapidly growing or indolent. This is the kind of individual factor that will influence the likelihood of a patient being in a position to benefit from this treatment. The trial report does not comment on the proportion of participants presenting to the trial centers that had treatable lesions. The verbal estimate from one trialist was approximately 25% and from another expert between 5% and 7%. This is crucial in estimating the impact of this new treatment on health-care budgets.
ARMD is a bilateral disease, although 1 eye is usually affected before the other. With a lesion present in 1 eye,
the annual cumulative incidence of a lesion in the second eye is estimated to be about 15%. Clinicians now commonly advise patients with a lesion in 1 eye to watch for the onset of symptoms in the second eye and to seek treatment as soon as they notice the symptoms to improve the chances of catching the lesion in the second eye in time. This often entails the provision of an Amsler grid, a simple chart on which a number of gridlines are printed around a central fixation spot. The patient is instructed to examine the grid and to look for focal distortion of the lines in the grid that would indicate local elevation of the retina as a result of the growth of an underlying membrane. This strategy offers the best hope of saving sight with this new treatment at least in places where access to a qualified ophthalmologist can be slow. It should also be recalled that this treatment does not restore sight but rather prevents further deterioration. Sustaining numerous assessments that involve relatively invasive treatments may have an adverse effect on the patient. Without patient-orientated outcomes in these trials, we cannot comment on the patient’s perspective on the experience of Visudyne therapy. It is likely that in most cases, especially where loss of sight of the second eye is threatened, patients would be willing to undergo all the necessary interventions, even when the probability of success is small. Quality-of-life outcomes have been independently reported in a cohort of individuals treated with PDT and followed for 1 year.13 There was no comparator group. At 12 months, participants were less anxious and more independent than baseline though there was a significant deterioration in more vision-related tasks. Adverse effects occurred infrequently with the exception of the rather vague “visual disturbance,” which affected more people in the verteporfin group compared with the control group. However, this was not reflected in the visualacuity outcomes. Infusion-related back pain occurred in 2.4%, substantially lower than in some other studies. For example, in a series of 250 people treated with verteporfin, 9.6% experienced verteporfin-associated pain, mostly back pain.25 It is now clear that acute severe visual-acuity decrease is a relatively small but serious risk of poor outcome of treatment. This review estimates this risk to be approximately 1 in 50 patients. The trials included in this review appear to have been performed to high standards and were closely supervised by the United States Food and Drug Administration. Both trials were sponsored by the manufacturers of the drug (CIBA Vision and Novartis Ophthalmics) and declared potential conflicts of interest existed for a number of the trialists who held interests in the manufacturer of the laser technology. This makes detailed scrutiny of reports of the trial essential. Of concern are the numerous protocol revisions that were registered with the Institutional Review Bodies throughout the study and after completion of follow-up. Although we have not had access to the mainprotocol or to the revisions, a CIBA representative had assured us that the changes were not substantive and, in particular, that there were no changes to the a priori determinants of the primary outcomes. New reviews have not drawn any conflicting conclusions or additional evidence. In particular, the review commissioned by the National Health Service’s Research and Development Health Technology Assessment Programme on behalf of the National Institute of Clinical Excellence (NICE) in the United Kingdom (www.nice.org.uk) was in accordance with the findings of our review but went on to perform a detailed cost and cost-utility analysis. They concluded through economic modelling that the benefits of PDT with verteporfin at 2 years were “at best at the margins of what is generally considered to be an efficient use of health-care resources.” Another paper from Australia (Hopley 2004)11 examined cost utility for PDT for predominantly classic neovascular ARMD using data from the TAP trial in 2 costutility models for 2 case scenarios. They concluded that, as the only available treatment for some forms of neovascular ARMD, PDT can be considered moderately cost-effective for those with reasonable acuity but less so for those with poorer presenting vision. These conclusions depend upon the validity of the subgroup analyses of the TAP trial and there must be some concern regarding one of the conclusions of the trialists’ post hoc analyses—that those with poorer presenting vision fare better in terms of number of lines of visual acuity lost. The NICE review concluded that there was still much uncertainty about the effectiveness of this treatment. In the face of enormous pressure to provide something that might work when nothing else is available, provision of service conditional on close monitoring of outcomes is a pragmatic approach, though implementation of this policy is difficult.
REVIEWERS’ CONCLUSIONS
Implications on practice
This review provides evidence that PDT in patients with classic and occult CNV due to ARMD is probably
effective in preventing visual loss though the size of the effect remains in doubt. On the basis of existing evidence, 7 people need to be treated with an average of 5 treatments over 2 years to prevent 1 person from losing 3 or more lines of visual acuity. One in every 50 treated patients will have an acute severe loss of vision in the treated eye. For an expensive treatment, there are questions about the cost-utility and indeed opportunity cost for health services, especially when resources are limited. Two trials were included in this review. Both trials were performed by the same investigators using largely the same clinical centers and funded by manufacturers of verteporfin. As for all new technology, outcomes and potential adverse effects need to be monitored when introduced into clinical practice and this recommendation has been implemented in the UK by the establishment of a national cohort study to monitor outcomes of verteporfin PDT according to NICE guidelines in the NHS. There are major implications for health services, both in terms of potential expenditure and organization, if PDT is to be introduced. Where referral to an ophthalmologist is through a primary-care network, facilities for the recognition of this condition in its early stages are needed. There is potential for an enormous increase in referral of people with early age-related maculopathy for assessment, in case an early treatable lesion is present. This could swamp already overstretched facilities at the secondarycare level. Extra resources will be required at the secondary-care level to manage increased referrals, for the necessary technology to diagnose treatable lesions and to deliver treatment.
Implications on research
Further independent trials of verteporfin are required to establish that the effects seen in this study are consistent and to examine important issues not yet addressed, particularly relating to quality of life and cost. A similar recommendation was made by the authors commissioned for NICE for publicly funded pragmatic
trials with economic and vision-related quality-of-life outcomes over a longer time scale. To our knowledge
no such studies are underway. Some commentators argue that technology is progressing at a pace that will render such studies irrelevant. New interventions for ARMD, particularly those based on drugs active against Vascular Endothelial Growth Factor, show some promise and there is speculation that the role of PDT-based treatments will be short-lived. Descriptive epidemiology on the population at risk and the numbers likely to benefit from these kinds of interventions remain essential to estimate the impact of these new treatments on health-service resources and the well-being of the ageing population of more affluent countries with a life expectancy sufficient to render ARMD a significant public-health concern. A particular concern remains that people in need of treatment can access it equitably and in time. Health services research of this nature and surveillance for rare but severe adverse effects are required.
References
1. Macular photocoagulation study group. Laser photocoagulation for juxtafoveal choroidal neovascularization: five year results from randomized clinical trials. Arch Ophthalmol 1994; 112: 500-509.
2. Higgins JPT, Green S, eds. Locating and selecting studies for reviews. Cochrane Handbook for Systematic Reviews of Interventions 4.2.4 [updated March 2005]; Section 5. In: The Cochrane Library, Issue 2, 2005. Chichester, UK: John Wiley & Sons, Ltd.
3. Higgins JPT, Green S, eds. Assessment of quality. Cochrane Handbook for Systematic Reviews of Interventions 4.2.4 [updated March 2005]; Section 6. In: The Cochrane Library, Issue 2, 2005. Chichester, UK: John Wiley & Sons, Ltd.
4. Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: One-year results of 2 randomized clinical trials – TAP report. Arch Ophthalmol 1999; 117: 1329-1345.*
4a. Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: Two-year results of 2 randomized clinical trials – TAP report 2. Arch Ophthalmol 2001; 119: 198-207.
4b. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Verteporfin therapy for subfoveal choroidal neovascularization in agerelated macular degeneration: three-year results of an open-label extension of 2 randomized clinical trials – TAP report no 5. Arch Ophthalmol 2002; 120:1307-1314.
5. Verteporfin in Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization – Verteporfin in Photodynamic Therapy Report 2. Am J Ophthalmol 2001; 131: 541-560.*
5a. Bressler NM. Verteporfin therapy of subfoveal choroidal neovascularization in agerelated macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization – Verteporfin In Photodynamic Therapy Report 2. Am J Ophthalmol 2002; 133: 168-169.
6. Rubin GS, Bressler NM, the Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Effects of verteporfin therapy on contrast sensitivity: results from the treatment of age-related macular degeneration with photodynamic therapy (TAP) investigation – TAP report no. 4. Retina 2002; 22: 536-544.
7. Schmidt-Erfurth UM, Elsner H, Terai N, et al. Effects of verteporfin therapy on central visual field function. Ophthalmology 2004; 111: 931-939.
8. Schmidt-Erfurth UM, Michels S. Changes in confocal indocyanine green angiography through two years after photodynamic therapy with verteporfin. Ophthalmology 2003; 110:1306-1314.
9. Meads C, Hyde C. Photodynamic therapy with verteporfin is effective, but how big is its effect? Results of a systematic review. Br J Ophthalmol 2004; 88: 212-217.
10. Azab M, Benchaboune M, Blinder KJ, et al. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: meta-analysis of 2-year safety results in three randomized clinical trials: Treatment Of Age-Related Macular Degeneration With Photodynamic Therapy and Verteporfin In Photodynamic Therapy Study Report no. 4. Retina 2004; 24:1-12.
11. Hopley C, Salkeld G, Mitchell P. Cost utility of photodynamic therapy for predominantly classic neovascular age related macular degeneration. Br J Ophthalmol 2004; 88: 982-987.
12. Arnold JJ, Blinder KJ, Bressler NM, et al. Acute severe visual acuity decrease after photodynamic therapy with verteporfin: case reports from randomized clinical trialsTAP and VIP report no. 3. Am J Ophthalmol 2004; 137: 683-696.
13. Armbrecht AM, Aspinall PA, Dhillon B. A prospective study of visual function and quality of life following PDT in patients with wet age-related macular degeneration. Br J Ophthalmol 2004; 88: 1270-1273.
14. Barbazetto I, Burdan A, Bressler NM, et al. Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin: fluorescein angiographic guidelines for evaluation and treatment-TAP and VIP report No. 2. Arch Ophthalmol 2003; 121:1253-1268.
15. Blinder KJ, Bradley S, Bressler NM, et al. Effect of lesion size, visual acuity, and lesion composition on visual acuity change with and without verteporfin therapy for choroidal neovascularization secondary to age-related macular degeneration: TAP and VIP report no. 1. Am J Ophthalmol 2003; 136:407-418.
16. Bressler NM, Arnold J, Benchaboune M, et al. Verteporfin therapy of subfoveal choroidal neovascularization in patients with age-related macular degeneration: additional information regarding baseline lesion composition’s impact on vision outcomes-TAP report No. 3. Arch Ophthalmol 2002; 120:1443-1454.
17. Bressler SB, Pieramici DJ, Koester JM, Bressler NM. Natural history of minimally classic subfoveal choroidal neovascular lesions in the treatment of age-related macular degeneration with photodynamic therapy (TAP) investigation: outcomes potentially relevant to management—TAP report No. 6. Arch Ophthalmol 2004;
122:325-329.
18. Woodburn KW, Engelman CJ, Blumenkranz MS. Photodynamic therapy for choroidal neovascularization: a review. Retina 2002; 22: 391-405.
19. Miller JW, Schmidt-Erfurth U, Sickenberg M, et al. Photodynamic therapy with verteporfin for choroidal neovascularisation caused by age-related macular degeneration. Results of a single treatment in a phase 1 and 2 study. Arch Ophthalmol 1999; 117: 1161-1173.

