Intravenous methylprednisolone versus oral prednisone for initial attacks of optic neuritis, A review of evidence
Teresita R. Castillo, MD, MHPEd
OPTIC neuritis, an inflammatory disorder of the optic nerve, has been cited as the most common optic neuropathy in adults, particularly individuals below 46 years of age.1 The common presentation of acute optic neuritis is that of an isolated clinical event manifesting with periocular pain, abnormal decrease in visual function, a relative afferent pupillary defect and abnormal electrophysiologic optic-nerve findings. Fundus findings range from a normal appearance of the optic nerve to opticnerve-head edema (papillitis). Using magnetic resonance imaging, changes in the form of white matter abnormalities similar to those in multiple sclerosis are seen in 50 to 70% of patients with monosynaptic optic neuritis.1 Steroids have long been used in the treatment of optic neuritis. Their effectiveness has been assessed utilizing clinical outcomes, particularly their effect on improving the visual function of patients. However, due to the high cost of intravenous methylprednisolone in the Philippines, local ophthalmologists often use oral-steroid preparations instead. It is the objective of this paper to review current available evidence that addresses the efficacy of intravenous methylprednisolone compared with oral steroids in improving vision among patients with optic neuritis.
SEARCH METHOD
An electronic literature search covering the years from 1980 to 2005 was performed using Medline (PubMed).
The key words utilized were “optic neuritis,” “steroids,” and “vision.” The search was further limited to randomized controlled trials, metaanalysis, or reviews published in the English language. Free-text and MeSH search methods were employed to maximize the number of hits. Table 1 presents the search process performed. Titles and abstracts identified from items 5, 7, 8, and 9 in Table 1 were reviewed for appropriateness in answering the clinical question. Twenty-three citations listed among the randomized controlled trials were considered appropriate to answer the clinical question. Most of these were reports concerning the results of the Optic Neuritis Treatment Trial (ONTT), a multicenter, randomized controlled trial sponsored by the National Eye Institute of the United States National Institutes of Health. 2-11, 13-16
Only 1 metaanalysis and 5 reviews from the search qualified. Of these, only 2 were retrieved for further
evaluation.1, 12 It is noteworthy that the metaanalysis and reviews made primary reference to the ONTT.
The first major article on the results of this landmark study published in 199211 and the follow-up reports on
the five-year and ten-year visual outcomes published in 1997 and 2004 were reviewed and appraised for purposes of answering the clinical question.2, 15 Additional information regarding specific aspects of the trial was likewise referred to when necessary.
CITATION
Beck RW, Cleary PA, Anderson MM, et al. A randomized controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med 1992; 326: 581-588.
Follow-up reports
1. The Optic Neuritis Study Group. Visual function 5 years after optic neuritis: experience of the Optic Neuritis
Treatment Trial. Arch Ophthalmol 1997; 115: 1545-1552.
2. Beck RW, Gal RL, Bhatti MT, et al. The optic neuritis study group. Visual function more than 10 years after optic neuritis: experience of the Optic Neuritis Treatment Trial. Am J Ophthalmol 2004; 137: 77-83. Erratum in: Am J Ophthalmol 2004; 137: following 793. Am J Ophthalmol 2004;138: following 321. Study characteristics of the Optic Neuritis Treatment Trial The study involved 457 patients who met the following inclusion criteria: between 18 and 46 years old, history consistent with acute unilateral optic neuritis, visual symptoms lasting 8 days or less, evidence of a relative pupillary defect and associated with visual-field defect. Patients with history of previous optic neuritis or ophthalmoscopic signs of optic atrophy and clinical evidence of systemic diseases other than multiple sclerosis causing the optic neuritis were excluded from the study. Eligible patients were randomly assigned to one of three groups: intravenous methylprednisolone (IVMP) (n=151), oral
prednisone (n=156), or placebo (n=150). The IVMP group received 250 mg of methylprednisolone intravenously every 6 hours for 3 days. This was followed by oral prednisone at a dose of 1 mg per kilogram of body weight per day for 11 days. The oral-prednisone group received prednisone at a dose of 1 mg per kilogram of body weight per day for 14 days and the placebo group received oral placebo on the same schedule as the oral prednisone group. Patients in both oral groups received

their treatment as a single morning dose. The dose was subsequently reduced to 20 mg on day 15 and 10 mg on days 16 and 18 for both prednisone treatment arms.11 The primary outcome measures were visual fields and contrast sensitivity. Visual acuity and color vision were secondary measures. Subjects were also monitored for occurrence of new attacks in either eye and the development of multiple sclerosis.
DISCUSSION
Validity criteria
The Optic Neuritis Treatment Trial was first appraised for validity in addressing the following issues:
Selection bias. Patients were randomly assigned in equal numbers to the 3 treatment groups. Allocation concealment was attained by using permuted blocks with a separate sequence for each clinical center. Adverse effects and associated events were recorded at each visit.11 In all 3 groups, patients were predominantly female Caucasians. Age distribution was also similar for all groups with a median of 32 years. Median weight did not differ significantly among the 3 treatment groups. Clinical characteristics described included duration of visual symptoms, presence of ocular pain, and optic-disk swelling. Baseline measurements of visual function (contrast sensitivity, visual-field deviation, visual acuity, and color vision) of patients did not differ significantly
among the 3 groups. The number of patients diagnosed with multiple sclerosis was, however, lower for the IVMP group (3 patients) compared with the oral-prednisone and placebo groups (7 patients each). In terms of patient characteristics, the 3 groups did not differ significantly.8 Performance bias. Both subjects and outcome assessors were blinded as to the treatment received in the oralintervention arms of the study. The IVMP arm was not blinded since the subjects assigned to this group required
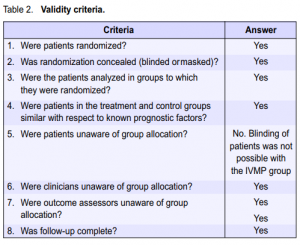
hospitalization and were, therefore, aware of the treatment they were receiving. While it would have been ideal to have a direct comparison between IVMP and IV placebo, administration of placebo via the IV route was not possible for ethical reasons. Nevertheless, efforts were also made to mask the outcome assessors in the evaluation of the IVMP group.11 Thus, every effort was taken to ensure that performance bias was kept to a minimum. Exclusion bias. The authors of the study were able to account for all patients for the duration of the study. Mention was made of exclusion of 2 patients after randomization because of misdiagnosis, but they were included in the data analysis based on their original group assignment. There were 26 dropouts by the end of the six-month period, 8 in the oral prednisone group and 9 each for the placebo and IVMP groups.11 The dropout rate, however, was below the acceptable limit of 20%. At the conclusion of the trial in 1992, 410 patients consented to continue follow-up until 1997. Beyond 1997, 387 patients consented to continue follow-up to complete the ten-year period. The five- and ten-year reports published by the Optic Neuritis Study Group were based on results from this cohort of patients. The ten-year report on visual recovery stated that of the 387 patients who gave their consent, examination was completed for 319 patients (82%). The status of the 135 original ONTT patients who did not complete the ten-year examination was, however, accounted for. Comparison of the characteristics of the 319 patients who completed the examination with the 135
patients who did not showed that they were similar with respect to age, sex distribution, proportion with abnormal baseline brain magnetic-resonance-imaging results, and proportion of patients with multiple sclerosis at baseline. Patients not completing the examination were, however, more likely to be African–Americans and, on average, had slightly worse acuity in the affected eye at baseline.2 Since all patients were accounted for by the investigators, exclusion bias was, therefore, kept to a minimum. Table 2 presents a summary of the answers to the various validity criteria set for treatment trials. Despite incomplete masking, it is safe to conclude that the study was valid.
Results of the study
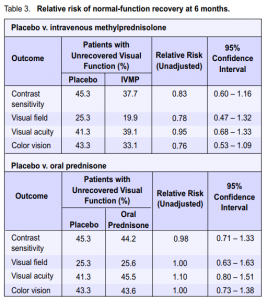
In the data analyses, each steroid group was compared with placebo, but no direct comparison between the 2
steroid regimens was made. Visual function. The relative risk for nonrecovery of visual function for the different outcomes is presented in Table 3. A relative risk (RR) less than 1.0 would be indicative of benefit from the intervention (steroid) while a relative risk greater than 1.0 would imply harm. Stratified results (based on visual function at the time of study entry) can be obtained from the original article. The visual outcomes in the IVMP group seemingly showed better chances of recovery (point estimates) compared with those in the placebo group. The oralprednisone group showed no benefit or reduction in risk in all outcome measures, except in contrast sensitivity, compared with the placebo group. These trends were, however, satistically insignificant based on the confidence intervals. In a separate report that evaluated correlation between several parameters and factors predictive of visual recovery, student t-test and least squares regression demonstrated that baseline visual acuity was a statistically significant predictor of the six-month visual acuity (Table 4). However, most patients with severe initial visual loss eventually had good recovery of vision, implying that the significance was more statistical than clinical.7 Patients who consented to participate beyond the original study duration were examined in the fifth and tenth year posttreatment.2, 15 Visual-function tests in the fifth year were normal or slightly abnormal in the affected and fellow eyes of most patients. Contrast sensitivity was frequently abnormal compared with the other visualfunction parameters in affected eyes. There was minimal change in vision from the sixth-month to the fifth-year exam.6, 7, 9 There was no significant difference in visual function after 5 years among the 3 treatment groups. Most patients retained good to excellent vision in the tenth year, with normal or slightly abnormal visualfunction-test results. There was no significant difference
in visual function among the 3 treatment groups.

Recurrence of optic neuritis.
The ONTT results showed that while the risk of recurrence for the affected eye did not differ significantly between the placebo and oral-prednisone groups, it was significantly increased for the contralateral eye. Comparing the risk of recurrence in either eye also showed a significantly higher risk for the oral-prednisone
group (Table 5). Comparisons between the placebo and IVMP groups revealed insignificant differences in risk of recurrence for either the affected or contralateral eye as supported by the confidence intervals. The five-year results reported the cumulative probability of having a new episode of optic neuritis during the 5 years of follow-up at 19% for the affected eye, 17% for the fellow eye, and 30% for either eye. In consonance with
CI – confidence interval
IVMP – intravenous methylprednisolone
MS – multiple sclerosis
the data reported from the two-year follow-up period, the probability of a new attack in either eye in the oralprednisone group was almost twofold (41%) that in either the placebo or intravenous group (25%). In addition, 14% of the patients in the oral-prednisone group had more than one episode in either eye compared with 7% in both the placebo and IVMP groups.15 Among the patients who completed the ten-year followup examination, 35% had a documented recurrence of optic neuritis in the affected eye (at study entry), at least
1 attack of optic neuritis in the fellow eye, or both after entry into the ONTT. The proportion of patients with a
recurrence in either eye was significantly higher in the oral-prednisone group (44%) than in the IVMP (29%, p = 0.03) or placebo group (31%, p = 0.07).2
Development of multiple sclerosis.
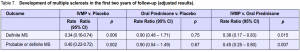
Within the 6 to 24 months follow-up period, multiple sclerosis developed in 20% (28 patients) in the placebo group, 14% (20 patients) in the intravenous group, and 24% (35 patients) in the oral-prednisone group.11 While these results showed that administration of intravenous methylprednisolone tended to have some beneficial effect in preventing the development of multiple sclerosis and oral-prednisone intake showed some tendency to harm, they were not significant as evidenced by the confidence intervals (Table 6). A separate report that focused on the development of multiple sclerosis within the first 2 years among patients
enrolled in the ONTT, compared the IVMP and oralprednisone groups with placebo and with each other
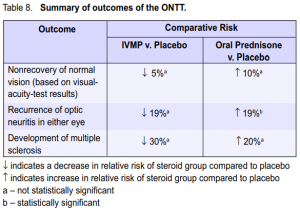
(Table 7). The adjusted results favored the IVMP group, which showed a reduced rate of development of multiple sclerosis.8 The latest report noted that the overall risk of developing clinically definite multiple sclerosis was 38% (95% CI 33% – 43%) over 10 years and 40% (95% CI 35% – 45%) over 12 years from the initial attack of optic neuritis. The ten-year risk was, however, similar in the 3 original ONTT treatment groups.3 Table 8 summarizes the results of the study. Except for the results comparing the percentage of patients who developed new episodes of optic neuritis between the oral prednisone and placebo groups, the rest of the results were insignificant. It should be noted, however, that the probability of nonrecovery of vision, having a new episode of optic neuritis, or development of multiple sclerosis was lower in the IVMP group than in the oral-prednisone group.
Side effects.
Reported side effects were generally mild. Serious side effects were reported in only 2 patients, both
in the IVMP group. One patient had an acute transient depression that required treatment and another had acute pancreatitis. Both cases resolved without sequelae. Minor side effects were more common in the 2 steroid groups than in the placebo group. These included sleep disturbance, mild mood change, stomach upset, and facial flashing. The mean percentage of weight gain was also higher in the steroid groups than in the placebo group (p < 0.001).11
STUDY AUTHORS’ CONCLUSIONS
In most patients with optic neuritis, recovery of vision is rapid—within 2 to 3 weeks after the onset of symptoms even without treatment. The only factor of value in predicting the visual outcome is the initial severity of vision loss. However, even when initial loss is severe, recovery of vision is still good in most patients. In the ONTT, treatment with high-dose intravenous methylprednisolone followed by oral prednisone accelerated recovery of vision but provided no long-term benefit to vision. Most patients retained good to excellent vision 5 to 10 years following an attack of optic neuritis regardless of the type of treatment they received.2, 11, 15 The probability of a new attack of optic neuritis in either eye was higher in the oral-prednisone group than in the other 2 groups. The probability of recurrence in either eye was greater among patients in whom clinically definite multiple sclerosis was diagnosed by the fifth and tenth
year of follow-up.2, 11, 15 Treatment with high-dose intravenous methylprednisolone followed by oral prednisone produced a short-term reduction in the rate of development of clinically definite multiple sclerosis, but there were no significant differences among treatment groups in either the risk of development of clinically definite multiple sclerosis or in the degree of neurologic disability among patients who developed
clinically definite multiple sclerosis 5 or 10 years following the initial attack of optic neuritis. 2, 11, 15 Intravenous methylprednisolone was generally well tolerated. Only 2 patients reported serious side effects, both cases of which resolved without sequelae.11
REVIEWER’S CONCLUSIONS
Intravenous methylprednisolone should be considered for use in patients with acute optic neuritis if there is a
need to speed up recovery of vision. Since long-term visual outcomes were comparable in the 3 treatment groups, and the oral-prednisone regimen, in doses given in this trial, was found to be associated with an increased risk of recurrence of optic neuritis in either eye, no treatment is an option for patients with an initial attack of optic neuritis. Baseline MRI studies should ideally be done for patients suspected of having optic neuritis in order to assess their odds of developing clinically significant multiple sclerosis. The clinical applicability of study results should be considered from the point of view of patient characteristics, values, and preferences. The cost of a three-day course of IVMP should be considered in treating any patient with optic neuritis in the local setting. Studies utilizing oral prednisone at doses other than that used in
the ONTT study may be explored as alternative treatment. In recent years, results of a trial comparing the administration of interferon with IVMP have been published. These should also be reviewed.
References
1. Kaufman DI, Trobe JD, Eggenberger ER, Whitaker JN. Practice parameter: the role of corticosteroids in the management of acute monosymptomatic optic neuritis. Report of the quality standards subcommittee of the American Academy of Neurology. Neurology 2000; 54: 2039-2044.
2. Beck RW, Gal RL, Bhatti MT, et al. The optic neuritis study group. Visual function more than 10 years after optic neuritis: experience of the optic neuritis treatment trial. Am J Ophthalmol 2004; 137: 77-83. Erratum in: Am J Ophthalmol 2004; 137: following 793. Am J Ophthalmol 2004; 138: following 321.
3. Beck RW, Gal RL, Bhatti MT, et al. The optic neuritis study group. High- and lowrisk profiles for the development of multiple sclerosis within 10 years after optic neuritis: experience of the optic neuritis treatment trial. Arch Ophthalmol 2003; 121: 944-949.
4. Beck RW, Trobe JD. What we have learned from the optic neuritis treatment trial. Ophthalmology 1995; 102: 1504-1508.
5. Beck RW, Trobe JD. The optic neuritis study group. The optic neuritis treatment trial: putting the results in perspective. J Neuroophthalmol 1995; 15: 131-135.
6. Beck RW. The optic neuritis treatment trial: three-year follow-up results. Arch Ophthalmol 1995; 113: 136-137.
7. Beck RW, Cleary PA, Backlund JC. The course of visual recovery after optic neuritis: experience of the optic neuritis treatment trial. Ophthalmology 1994; 101: 1771- 1778.
8. Beck RW, Cleary PA, Trobe JD, et al for the optic neuritis study group. The effect of corticosteroids for acute optic neuritis on subsequent development of multiple sclerosis. N Engl J Med 1993; 329: 1764-1769.
9. Beck RW, Cleary PA. Optic neuritis treatment trial: one-year follow-up results. Arch Ophthalmol 1993; 111: 773-775.
10. Beck RW, Kupersmith MJ, Cleary PA, Katz B. Fellow eye abnormalities in acute unilateral optic neuritis: experience of the optic neuritis treatment trial. Ophthalmology 1993;100: 691-697; discussion 697-698.
11. Beck RW, Cleary PA, Anderson MM Jr, et al. The optic neuritis study group. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med 1992; 326: 581-588.
12. Brusaferri F, Candelise L. Steroids for multiple sclerosis and optic neuritis: a
metaanalysis of randomized controlled clinical trials. J Neurol 2000;247: 435-442.
13. Keltner JL, Johnson CA, Spurr JO, Beck RW. Visual field profile of optic neuritis: one-year follow-up in the optic neuritis treatment trial. Arch Ophthalmol 1994; 112: 946-953.
14. Optic neuritis study group. The clinical profile of optic neuritis: experience of the optic neuritis treatment trial. Arch Ophthalmol 1991; 109: 1673-1678.
15. Optic neuritis study group. Visual function 5 years after optic neuritis: experience of the optic neuritis treatment trial. Arch Ophthalmol 1997; 115: 1545-1552.
16. Trobe JD, Beck RW, Moke PS, Cleary PA. Contrast sensitivity and other vision tests in the optic neuritis treatment trial. Am J Ophthalmol 1996; 121: 547-553.

