4-Year Prevalence and Outcome of Initial Screening for Retinopathy of Prematurity in a Tertiary Hospital
James Rommet D. Luz, MD and Marcelino Banzon, MD
Retinopathy of prematurity (ROP), previously called retrolental fibroplasia, is a proliferative retinopathy of premature and low birth weight infants1. Premature birth triggers the onset of ROP, in which normal retinal vascular development is altered and abnormal neovascularization occurs,2 hence its biphasic disease. The initial phase of vessel growth retardation occurs from birth to postmenstrual age of approximately 30–32 weeks which leads to tissue hypoxia. The second phase is the vessel proliferation characterized by hypoxia-induced retinal neovascularization3. Retinopathy of prematurity is divided into 5 stages, stage 5 being the most severe.
Retinopathy of prematurity (ROP) is a major cause of blindness in children in the developing and developed world despite current surgical and laser treatment in the late-stage of the disease3. Potentially avoidable causes of blindness in children and the rate of blindness from ROP vary greatly among nations, being influenced both by levels of neonatal care (in terms of availability, access, neonatal outcomes) and by the availability of effective screening and treatment programs4. The World Health Organization (WHO) reported that approximately 2/3 of the estimated 1.5 million cases of blindness in children worldwide is in Asia.5 Among the leading causes of blindness were corneal opacification caused by measles, xerophthalmia, use of traditional eye medicine, optic nerve atrophy, amblyopia, infantile glaucoma, cortical visual impairment, and retinopathy of prematurity. In the Philippines, a projected 100 children will lose their eyesight per week; nearly 50% of these cases are either treatable or preventable.6 Leading causes are vitamin A deficiency, malnutrition, measles, and premature birth. The importance of ROP screening has dramatically increased in recent years as clinical studies have shown improved visual outcomes in infants with severe ROP after treatment with either transscleral cryotherapy or transpupillary laser therapy.
A new set of guidelines for screening and treatment have been published by the American Academy of Pediatrics (AAP) in 2006 and by the Royal College of Ophthalmologists and the Royal College of Paediatrics and Child Health, United Kingdom, in 2008. The studies suggested that ROP screening should be performed at 31 weeks postmenstrual age in infants with gestational ages of 26 6/7 weeks or less at birth, and at four weeks chronological age in infants with gestational ages of 27 weeks or more, or birth weights of 1250 gm or less at birth by an ophthalmologist skilled in the detection of ROP5. This guideline is being followed by the Nursery Unit of the Department of Pediatrics, University of Santo Tomas Hospital. The Department of Ophthalmology follows the International Classification of ROP (ICROP) which described vascularization of the retina and characterized ROP by its position (zone), severity (stage), and extent (clock hours).
An unpublished study by Ladores in 2008 showed no correlation between body weight and age of gestation with ROP staging. However, the results were obtained from a limited number of patients screened, of which only a total of 10 patients (20 eyes) met the inclusion criteria within the 1-year period. The authors recommended that a similar study be done using a larger population and a longer study period. This study, therefore, determined the 4-year prevalence of ROP on initial examination of newborn infants referred for ROP screening. The study also correlated the birth weight and age of gestation with ROP staging, and determined the number of ROP patients who underwent treatment and their status on follow-up.
METHODOLOGY
Medical records of infants screened for ROP from August 1, 2008 to July 31, 2012 at the University of Santo Tomas Hospital Clinical Division were reviewed. Infants with birth weights of 1500 grams or less and gestational age of 32 weeks or less and referred to the Department of Ophthalmology for ROP screening were included. Infants born with gestational ages beyond 32 weeks and birth weights more than 1500 grams were excluded. Demographic data consisting of patient’s name, gender, birthdate, birth weight (BW) and age of gestation (AOG) at birth, postconceptional age, other risk factors such as prolonged oxygen exposure and difficulty weaning off oxygen, date of referral, ROP staging on initial examination, progression of the disease, treatment and their outcomes were collated and recorded.
Pediatric Ophthalmologists and Vitreo-Retina specialists
All included infants underwent pupillary dilatation and a careful funduscopic examination by indirect ophthalmoscopy with scleral depression. Initial screening was performed on infants 31 weeks postmenstrual age when born at gestational age of 26 6/7 weeks or less at birth or on infants four weeks’ chronological age with gestational age of 27 weeks or more at birth. Patients were examined by ophthalmology residents under the supervision of vitreoretina specialists. Findings on examination were recorded on standard data sheets, noting whether or not plus disease was present, and the ROP stage determined with its corresponding location by zones and clock-hours in accordance with the accepted Clinical Practice Guidelines.
Data on the initial examinations and follow-ups were recorded. Prevalence was determined using frequencies and percentages. Correlation of ROP to age of gestation and birth weight were analyzed using chi square tests.
RESULTS
There were a total of 3396 births at the University of Santo Tomas Hospital Nursery Section from August 1, 2008 to July 31, 2012. Of these, 251 were preterm infants with gestational age of less than 37 weeks. Fifty-three from the 251 preterm infants met the criteria for ROP screening (Table 1). However, only 25 of these patients who were referred for ROP screening with strict compliance to the guidelines (BW ≤1500 grams or ≤32 weeks) were included in the study. The remaining patients (n=28) were not referred for screening due to the following reasons: 7 patients expired, 10 were transferred, 6 were discharged against medical advice, and 5 were not referred due to medical instability of the patient.
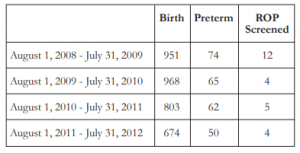
A total of 50 eyes from 25 infants, 9 boys and 16 girls, were screened for ROP. The mean post- conception age of referral for ROP screening was 3 6/7 weeks (1 week to 9 weeks). On initial examination, 9 eyes of 5 infants were positive for ROP for a prevalence of 18%. Of these, one patient had stage 4 zone II ROP with plus disease and visible macular dragging in the right eye and stage 3 zone II in the fellow eye. The eye with stage 4 ROP was advised to undergo surgery; however, due to severe pneumonia, the procedure was not done. Both eyes underwent laser photocoagulation whereby the left eye had notable regression of ROP. The right eye progressed to cicatricial ROP after re-evaluation and was monitored regularly. Additional two eyes (4%) had stage 3 ROP with plus disease in 1 infant referred at 4 weeks chronological age warranting laser intervention in both eyes and anti-vascular endothelial growth factor (anti-VEGF) injection in the right eye. Re-evaluation was done 1 week after with noted regression of ROP in both eyes. Five eyes (10%) of 3 infants had Stage 1 zone III ROP and was observed and followed up after 2 weeks with improvement. Thirty eyes (60%) of 15 infants had immature retina while 11 eyes (22%) of 6 infants had mature retina. The mean gestational age in weeks and the mean birth weight in grams are shown in Table 2.
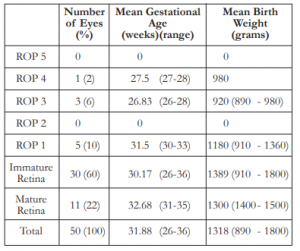
Of the total number of cases seen, the mean gestational age in weeks was 31.88 (26-36 weeks), with mean birth weight of 1318 grams (890 – 1800 grams). Of the 4 eyes with severe ROP requiring intervention, the mean AOG in weeks was 27, with mean BW of 935 grams. Cases with mild ROP (stage 1 and 2) showed a mean AOG in weeks of 31.5 and the mean BW of 1180 grams.
The frequency of ROP by gestational age in weeks is shown in Table 3. All cases with ROP had a gestational age of 32 weeks or less. Chi square correlation analysis showed significant relationship between AOG and ROP (χ2= 13.69, α = 9.49).
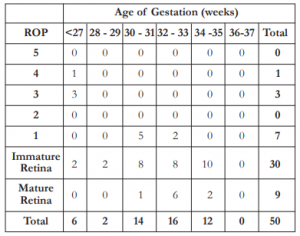
The frequency of ROP by birth weight in grams is shown in Table 4. All cases with ROP had a birth weight of 1360 grams or less. Chi square correlation analysis showed no significant relationship between BW and ROP (χ2= 6.50, α = 9.49).

Additional data obtained from the medical records included a positive history of exposure to oxygenation by CPAP, NCPAP, or mechanical ventilation in 9 (100%) infants, sepsis in 7 (78%), and blood transfusion in 4 (44%).
Ten eyes (20%) of 5 patients had follow-up evaluation. Three eyes of two infants who had stage 3 with plus disease and underwent laser photocoagulation and 1 eye that underwent anti- VEGF injection after the initial screening showed regression of ROP. One eye of 1 infant with ROP stage 4 with plus disease showed stable ROP on repeat examination. Six (12%) eyes of 3 infants, 1 (2%) with ROP stage 1 zone III and 5 (10%) with immature retina collectively showed maturity of the retina upon re-examination.
DISCUSSION
The progression of retinopathy of prematurity involves 2 phases: the hyperoxia-vasocessation (phase 1) and the hypoxia-vasoproliferation (phase 2). Currently, the American Academy of Ophthalmology still uses the International Classification of ROP (ICROP) which describes the disease by stage, zone, and extent.7 Our department also uses this classification for identification, staging of ROP, and decision making in ROP management.
For the past 4 years, our hospital was able to identify 9 eyes (18%) of 5 infants who were positive for ROP out of 50 eyes that were included in the study. There was almost equal prevalence of ROP per year. Two eyes (4%) of 2 infants that were determined to have ROP stage 3 with plus disease immediately had laser ablative therapy while the other eye of another patient (2%) underwent both laser ablative therapy and anti-VEGF injection, all of which regressed on follow-up evaluation. On further review, one patient was referred for screening at 9 weeks after birth (approximately 36 weeks postmenstrual age) and was determined to have stage 4 ROP in the right and stage 3 in the left with plus disease in both eyes. The late referral was due to the instability of the medical condition, underscoring the fact that the patient’s medical condition may affect the timing of the referral. Decision on laser therapy was patterned in accordance with the Early Treatment for ROP Randomized Trial study9 where findings of threshold ROP was not the only preferred indication. Treatment may also be initiated for: 1) Zone I ROP: any stage with plus disease, 2) Zone I ROP: no plus disease, and 3) Zone II: stage 2 or 3 with plus disease. Treatment should generally be accomplished within 72 hours of determination of treatable disease to minimize the risk of retinal detachment.9 Five eyes of 6 infants developed stage 1 zone III ROP and were observed during their hospital stay. On follow-up, all eyes improved.
Comparing this data to the prevalence of ROP in a developed country like the United States, the total ROP among premature infants was 15.58% from 1997 to 2005.10 The study conclusively demonstrated the importance of low birth weight as a risk for ROP development in infants. Data from the Middle East, a developing region, showed a prevalence of 19.2% from 2009 to 2010.11 In India, the prevalence was 19.7% from 2009 to 201012. Although the prevalence of ROP among developing countries was relatively higher than that of developed countries, the difference was minimal. This demonstrates that retinopathy of prematurity is a worldwide problem that needs to be recognized and addressed. The slight difference in prevalence may be explained by the following: Firstly, rates of preterm birth tend to be higher in developing than in developed countries where teenage pregnancies are common. Second, in developing countries the proportion of women who are delivered in health care facilities is high and premature babies are, therefore, likely to be admitted to neonatal intensive care. Third, rates of severe ROP are higher in premature babies in low and middle income countries even when wider screening criteria have been used, suggesting that babies are being exposed to risk factors which are now largely controlled in industrialized countries.
This study shows that only a relatively small percentage (8%) of the ROP positive cases on initial screening were severe enough to warrant immediate treatment when adherent to the recommended guidelines, a number that is lower compared to the 12% in a similar local study done by Cerdena.4 Moreover, the significance of following the screening criteria for ROP as an important step in decreasing the incidence of blindness secondary to ROP in the Philippines should be highlighted. Fierson and colleagues recommended that “infants with birth weight of less than 1500 grams or gestational age of 32 weeks or less and selected infants with a birth weight between 1500 and 2000 grams or gestational age of more than 32 weeks with an unstable clinical course, including those requiring cardiorespiratory support and who are believed by their attending pediatrician or neonatologist to be at high risk, should have retinal screening examinations performed after pupillary dilation using binocular indirect ophthalmoscopy to detect ROP”.8 Retinal examination among preterm infants should be performed by an ophthalmologist who has sufficient knowledge and experience to enable accurate identification of the location and sequential retinal changes of ROP. The initiation of acute-phase ROP screening should be based on the infant’s age. The onset of serious ROP correlates better with postmenstrual age (gestational age at birth plus chronologic age) than with postnatal age.Current literature indicated that low BW and prematurity are still the most important risk factors in the development of ROP. In our study, 5 eyes (10%) of 3 infants developed stage 1 zone III ROP with mean gestational age of 32 weeks (ranging 30 – 33) and mean birth weight of 1234 grams (ranging 910 – 1360). Four eyes (8%) of 2 infants who had severe ROP (Stage 3 – 4 with plus disease) had a mean gestational age of 27 weeks (ranging 26 – 28) and mean birth weight of 935 grams (890 – 980). Statistical analysis using chi square test showed no significant correlation between the development of ROP and birth weight. This may be due to a small number of subjects that were included. On the other hand, correlation between development of ROP and AOG showed an inverse relationship— an increase in age of gestation led to a decrease in the development of ROP.
Other factors should also be included in the screening of ROP that could possibly cause or contribute to the development of ROP. The association between ROP and excessive oxygen exposure was recognized shortly after the initial description of the disease3. In our study, 100% of premature infants who developed ROP were exposed to oxygen supplementation. A study evaluating the use of improved oxygenation monitors and avoidance of fluctuation in the fraction of inspired oxygen showed a reduction in the rate of severe ROP without increased morbidity and mortality.2 Several diseases were frequently seen in infants who developed ROP, specifically respiratory distress syndrome (RDS), congenital heart disease commonly patent ductus arteriosus, apnea, bradycardia, sepsis, anemia, and jaundice.2 Out of the 25 patients included in this study, 78% developed sepsis, 44% had blood transfusion, and 22% developed pneumonia, RDS, anemia, or had congenital heart disease.
Our study is limited by a small sample size screened for ROP. The subjectivity of ROP staging was dependent on the judgments of different retina specialists that examined the infants during the screening process. Thus, we recommend a longer term study with a larger sample size to accurately correlate the age of gestation and birth weight to the development of ROP, and to evaluate the different risk factors.
With increasing prevalence of retinopathy of prematurity worldwide, early recognition and treatment of this condition, including regular follow-ups, are vitally important to prevent severe blindness. There is a significant correlation between age of gestation and ROP staging. This study, however, showed no correlation between birth weight and ROP staging.
References
1. Regillo CD, Holekamp N, Johnson MW, et al. 2008-2009 Basic and Clinical Science Course (BCSC) Section 12: Retina and Vitreous. American Academy of Ophthalmology, Beach Street, San Francisco, CA: 2008; pp. 124-136.
2. Simon JW, Aazy AA, Drack AV, et al. 2009-2010 Basic and Clinical Science Course (BCSC) Section 6: Pediatric Ophthalmology and Strabismus. American Academy of Ophthalmology, Beach Street San Francisco, CA: 2009; pp. 325-332.
3. Chen J, Stahl A, Hellstrom A, Smith LE. Current update on retinopathy of prematurity: screening and treatment. Curr Opin Pediatr 2011;23:173-178.
4. Cerdana HGS, Liao CS, Macias EL, Nañagas MLR. Results of initial screening for retinopathy of prematurity at a tertiary hospital. Philipp J Ophthalmol 2010;35:56-60.
5. Jefferies AL; Canadian Paediatric Society, Fetus and Newborn Committee. Retinopathy of prematurity: Recommendations for screening. Paediatr Child Health 2010;15:667-670.
6. Philippine Pediatric Society Inc., Pediatric Blindness Prevention and Vision Screening. PPS Policy Statement: 2004; vol. 1, no. 6.
7. International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol 2005;123:991- 999.
8. Fierson WM, Flynn J, Good W; Section on Ophthalmology, American Academy of Pediatrics, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2006;117:572-576.
9. Early Treatment for Retinopathy Cooperative Group. Revised indication for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 2003;121:1684-1694.
10. Lad EM, Hernandez-Buossard T, Morton JM, Moshfeghi DM. Incidence of retinopathy of prematurity in the United States: 1997 through 2005. Am J Ophthalmol 2009;148:451- 458.
11. Hakeem AH, Mohamed GB, Othman MF. Retinopathy of prematurity: a study of prevalence and risk factors. Middle East Afr J Ophthalmol 2012;19:289-94.
12. Sharan R, Jah AK, Bhusan B, Nath S. Retinopathy of prematurity – experience from a secondary care center. Indian Pediatr 2012;49:675.
13. Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk, and implications for control. Early Hum Dev 2008;84:77-82.

