Fluorophotometric measurements of aqueous-humor flow in post-YAG laser iridotomy for acute angle closure
Karlo D. Jacob, MD, Ma. Margarita Lat-Luna, MD, Patricia M. Khu, MD, MS
PRIMARY-ANGLE-CLOSURE (PAC) attack is an acute onset of intraocular-pressure (IOP) elevation due to
pupillary blockage by the lens of aqueous-humor flow, resulting in anterior movement of the peripheral iris
against the trabecular meshwork. Laser iridotomy (LI) is the treatment of choice for primary angle closure
secondary to pupillary block. It is also recommended for the fellow eye that is at risk for developing angle closure since it shares the same anatomic predisposition. When the pupillary block is relieved by LI, the anterior chamber deepens and the peripheral iris is no longer against the trabecular meshwork, the pressure gradient between the anterior and posterior chambers approaches zero, the angles open up, and the IOP is lowered. There are instances, however, when the angles are open gonioscopically but the IOP remains elevated and additional glaucoma medications are needed. Thus, even though the angle structures are visible on gonioscopy, there may already be damage to the trabecular-outflow facility with consequent rise in IOP.
The rate of aqueous-humor flow through the anterior chamber is one of the major determinants of IOP,1, 3-4 An important development in the measurement of aqueous flow in humans was the invention of a technique for measuring the rate of clearance of topically applied fluorescein. Fluorophotometry is a method of measuring the concentration profile of the tracer fluorescein within the ocular cavity, to accurately monitor the dynamics of intraocular diffusion and elimination. The objective and quantitative capabilities of fluorophotometry permit the detection of physiological changes very early in the course of certain diseases, and the monitoring of progress in treatment. It is a successfully employed research tool in both laboratory and clinical settings. The Ocumetrics Fluorotron Master fluorophotometer is one such instrument that measures fluorescence inside the eye. It was originally designed to measure the leakage of fluorescein dye from the retina into the vitreous in the same way that fluorescein angiography photographs this leakage. In glaucoma studies, aqueous fluorophotometry is used to observe the flow rate of aqueous humor through the anterior chamber. The fluorophotometer has a computer algorithm for subtracting background and vascular fluorescence so that only penetrated fluorescein is measured. It measured the rate of aqueous humor flow in normal Filipino eyes, and the results obtained were similar to those reported in foreign literature.2 A fluorescent dye is instilled into the anterior chamber. This may be accomplished by placing a drop of 2% fluorescein dye on the cornea.1, 3 The fluorescent dye passes through the cornea into the anterior chamber. The changing concentration of the dye has in the past been determined by projecting light into the anterior chamber using a slitlamp and detecting the resultant corneal and aqueous fluorescence. The dye distributes itself evenly into the cornea within 15 to 20 minutes. During the first 2 to 3 hours, the concentration of fluorescein in the cornea falls as the concentration in the anterior chamber rises. The instrument projects a beam of blue light in the form of a vertical slit into the eye. At the same time, a detector filtered to allow only fluoresced light is focused on the same point in the eye. Only fluorescence at the intersection of the source-light path and the detector-light path will be recorded. The fluorescent compound of interest is measured at only that particular point in the eye. It was Maurice who first realized that the corneal stroma could serve as a depot from which fluorescein could be introduced slowly into the anterior chamber. Fluorescein is introduced into the cornea by applying a high concentration in the conjunctival cul-de-sac. Having penetrated the epithelium and entered the stroma, fluorescein, which is not metabolized in the eye, disappears in one of three ways. First, by rediffusing through the corneal epithelium and flowing away with the tears; second, by diffusing laterally into limbal tissue; and third, by penetrating the endothelium and entering the anterior chamber. The third pathway offers the least resistance and consequently is the major route of loss from the stroma. Once in the anterior chamber, the tracer is washed away by flowing aqueous or diffuses into the iris. However, this
diffusional loss in the iris has been shown to account for only less than 10% of the total clearance.5 Fluorophotometry also provides another way to assess outflow facility. Outflow facility is calculated by this equation: C = Flow rate/(IOP – episcleral venous pressure).6 This is based on the symbolic description of
steady-state aqueous dynamics known as the Goldmann equation. The aqueous-humor flow is measured by
fluorophotometry and the IOP is measured by applanation tonometry. This study determined the rate of aqueous-humor flow in eyes that have had primary-angle-closure attack, and used these values to compute for the trabecular-outflow facility. The results of the aqueous-humor flow and trabecular-outflow facility in these eyes were compared to those of the fellow, non-attack eye (PACS). The functionality of the trabecular-outflow facility was also compared with the degree of angle opening and angle configuration in both the PAC-attack and PACS eyes.
METHODOLOGY
This is a cross-sectional study conducted at Sentro Oftalmologico Jose Rizal of the University of the Philippines–Philippine General Hospital (UP–PGH) between February 2009 and September 2009. Subjects were recruited from the Glaucoma Clinic of the Department of Ophthalmology and Visual Sciences. All patients were
18 years old and above who had PAC attack on one eye and PACS on the fellow eye, both–treated with LI, and
have at least a quadrant of open angle on gonioscopy postlaser, were included in the study. PAC attack is an acute onset of sudden IOP elevation associated with intermittent/episodic blurring of vision, discomfort, frontal headaches, glare, and colored rings around lights. Slitlamp examination shows congested episcleral and
conjunctival vessels, corneal edema, mild cells and flare in the narrow anterior chamber, signs of pupillary dilation with minimal or absent reaction to light, and almost the entire angle is closed. PACS is defined as an eye in which appositional contact between the peripheral iris and posterior trabecular meshwork is present or considered possible. Other forms of glaucoma, such as chronic angle closure, primary open angle, uveitic, neovascular, traumatic, steroid-induced; history of any ocular laser or incisional surgery; persistent elevation of IOP > 30 mm Hg despite maximum glaucoma therapy; and any ocular condition precluding good visibility of the angles, such as extensive corneal opacities and corneal edema, were excluded. Those who fulfilled the criteria underwent a complete eye evaluation that included gonioscopy, ultrasonic pachymetry (Pocket II, Quantel Medical, France) to obtain the central corneal thickness (CCT), and A-scan (OTI Scan 1000, Ophthalmic Technologies Inc., Canada) to measure the anterior-chamber depth. All included eyes underwent fluorophotometry. Three drops of 2% fluorescein dye were instilled on both eyes every 5 minutes for 3 doses. Excess fluorescein were removed with balanced saline solution 15 minutes after administration. To ensure that the fluorescein in the anterior chamber was in steady state with the cornea, fluorophotometry was performed 4 hours after instillation. All measurements were performed at 10 o’clock in the morning. Three scans were obtained for each eye every 30 minutes for 2 hours. The rates of aqueous flow and aqueous-outflow facility were subsequently obtained for each eye. The following outcome measures were recorded: age, sex, IOP, central corneal thickness (CCT), anteriorchamber depth, anterior-chamber volume, rate of aqueous flow, and aqueous-outflow facility. The data were subjected to descriptive analysis. Onesample and two-sample t-tests were used to compare the mean differences of the fluorophotometric measurements of aqueous humor flow and aqueous outflow facility of the 2 groups. The mean values and 95% confidence intervals were computed. Two-tailed alternative hypothesis was also used and tests below 0.05 were regarded as statistically significant.
The study adhered to the Declaration of Helsinki and was approved by the Ethics Committee of UP–PGH. All
subjects signed the informed-consent form.
RESULTS
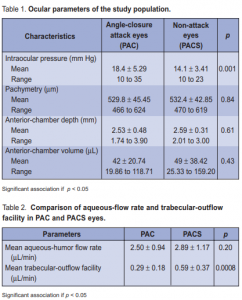
A total of 50 eyes of 25 patients were included in this study. Twenty-five eyes had an episode of PAC attack and the fellow eyes were diagnosed as PACS. The central corneal thickness (CCT), anterior-chamber depth, and
anterior-chamber volume between the 2 groups were comparable (Table 1). Eyes in the PAC-attack group,
however, had significantly higher IOP (18.4 mm Hg) than those in the PACS group (14.12 mm Hg) (p = 0.001).
The mean rate of aqueous-flow was 2.50 ± 0.94 µL/min in the PAC and 2.89 ± 1.17 µL/min in the PACS eyes. The difference, however, was not significant (Table 2). The mean aqueous-outflow facility was 0.29 ± 0.18 µL/min in the PAC and 0.59 ± 0.37 µL/min in the PACS eyes. Hence, eyes in the PAC group had significantly lower aqueousoutflow facility (p = 0.0008). The degree of angle opening and configuration were compared to the functionality of trabecular-outflow facility in both groups. PAC and PACS eyes were subdivided into 3 subgroups depending on the number of quadrants (2, 3, or 4) with open angles . In PACS, the trabecular-outflow facility among the subgroups were comparable (Table 3). In PAC, however, the subgroup with 4 open quadrants
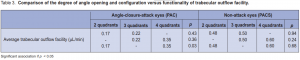
(0.35 µL/min) showed a significantly higher aqueousoutflow facility than those with 2 open quadrants (0.17 µL/min) (p = 0.03).
DISCUSSION
PAC attack is an ocular emergency and receives distinction due to its acute presentation, need for immediate
treatment, and well-established anatomic pathology. Acute angle closure is present with at least 2 of the following symptoms: ocular pain, nausea/vomiting, and a history of intermittent blurring of vision with halos; and at least 3 of the following signs: IOP >21 mm Hg, conjunctival injection, corneal epithelial edema, mid-dilated nonreactive pupil, and shallower chamber in the presence of occlusion. Primary angle closure is defined as an occludable drainage angle and features indicating that trabecular obstruction by the peripheral iris has occurred (i.e., peripheral anterior synechiae, increased IOP, lens opacities, excessive trabecular pigmentation or deposit). The term glaucoma is added if glaucomatous optic neuropathy is present. Studies that evaluated patients after treatment for PAC attack showed favorable outcomes. With adequate treatment, most patients recover their lost vision. Nd: YAG LI is effective in widening the drainage angle and reducing elevated IOP. In Caucasians, IOP was controlled with LI alone in 65 to 76% of cases.8 However, Asians more often have medically refractory initial attacks and require medications after LI.8-9 They also have higher rates of visual-field loss and subsequent increases in IOP. It has been hypothesized that the initial attack is often more severe in Asians, resulting in greater trabecular damage.8 This study supported the contention that eyes that suffered
previous angle-closure attacks generally had higher mean IOP even if their angles were gonioscopically open but some trabecular damage had already occurred necessitating additional glaucoma medications. In addition, the degree of angle opening after LI also determined the functionality of aqueous-outflow facility. Fluorophotometry is a noninvasive method of determining the rate of aqueous-humor flow. This flow is said
to peak in the morning, slightly decrease in the afternoon, and is lowest during sleep. These changes throughout the day reflect a biologic pattern. The normal rate ranges from 1.5 to 4.5 µL/min. The reported mean rate of aqueoushumor flow between 8 in the morning and noon is 2.97 ± 0.77 µL/min.Our results in PAC and PAC eyes were within the normal range. The difference between the two mean aqueous-flow rates was not significant (p = 0.20). Kashiwagi and associates10 demonstrated that eyes with PAC attack showed poorer IOP control after LI than eyes with PACS and LI. Nolan and Foster also hypothesized that angles that have had an acute attack of angle closure developed an outflow problem. Even if the angles were anatomically open, there already was some form of functional derangement. According to Sihota and colleagues,11 pigment accumulation in the trabecular spaces and within the cells, and attenuated endothelial cells with a noninflammatory degeneration appeared to be the primary changes in the trabecular meshwork. Excessive phagocytosis of foreign material by trabecular endothelial cells caused them to grow larger and desquamate.12 Repeated occurrence of such pigment phagocytosis has been associated with trabecular-cell loss. Pigment release during an acute attack of PAC could affect the number and the normal functions of the endothelial cells. One of the major functions that will be affected is the synthesis and maintenance of the surrounding connective tissue. Changes in the endothelial cells, therefore, could affect the compliance of the trabecular beams and alter the resistance to outflow. Our study clearly demonstrated that PAC-attack eyes have lower outflow facility than PACS in spite of anatomically open angles and higher IOPs. The degree of angle opening and configuration were also compared to the functionality of trabecular-outflow
facility. In this study, trabecular outflow facility among the subgroups in PACS were comparable. In the PAC, however, the subgroup with 4 open quadrants (0.35 µL/min) showed a significantly higher aqueous-outflow facility compared to those with only 2 open quadrants (0.17 µL/min). Prior iridotrabecular touch could have left residual iris tissues attached to the trabecular space that may not be visible on gonioscopy or the prolonged contact could have caused degenerative changes altering the outflow facility. Thus, gonioscopic evaluation of the extent of peripheral anterior synechiae may not truly reflect the extent of trabecular meshwork damage in PAC eyes. It has been observed that some eyes continue to have elevated IOPs, despite patent
iridotomy and reversal of iridocorneal apposition. In conclusion, there was a significantly lower aqueousoutflow facility among eyes in the PAC attack group as demonstrated by fluorophotometr y. Despite the anatomically open angles, they continue to have higher IOPs. It is important, therefore,that all patients with PAC attack have long-term follow-up.
References
1. Brubaker RF. Measurement of aqueous flow by fluorophotometry. In: Ritch R, Shields MB, Krupin T, eds. The Glaucomas 2nd ed. St Louis, Mo: CV Mosby Co, 1996; v. 1, chap 22: 447-454.
2. Comia G, Lat-Luna M, Agulto M. Fluorophotometric measurements of aqueoushumor flow in Filipino eyes. Philipp J Ophthalmol 2007; 32: 56-59.
3. Bloom JN, Levene RZ, Thomas G, Kimura R. Fluorophotometry and the rate of aqueous flow in man. I. Instrumentation and normal values. Arch Ophthalmol 1976; 94: 435-443.
4. Brubaker RF, Nagataki S, Townsend DJ, et al. The effect of age on aqueous-humor formation in man. Ophthalmology 1981; 88: 283-288.
5. Brubaker RF. Flow of aqueous humor in humans. Invest Ophthalmol Vis Sci 1991; 32: 3145-3166.
6. Toris CB, Camras CB. Measuring the outflow of aqueous humor. Glaucoma Today September 2007: http://bmctoday.net/glaucomatoday/2007/09/article.asp? f=GT0907_01.php (accessed September 2008).
7. Darkeh AK, Silverberg MA. Glaucoma: acute angle-closure. Emedicine August 12, 2009: http://emedicine.medscape.com/article/798811-overview (accessed October 2009).
8. Nolan W, Foster P, Devereux J, et al. YAG laser iridotomy treatment for primary angle closure in east Asian eyes. Br J Ophthalmol 2000; 84: 1255-1259.
9. Koay An, Lim AKE, Hussain R, Rahman RA. Long-term intraocular-pressure outcome following an attack of acute primary angle closure. Philipp J Ophthalmol 2006; 31: 29-34.
10. Kashiwagi K, Abe K, Tsukahara S. Quantitative evaluation of changes in anteriorsegment biometry by peripheral laser iridotomy using newly developed scanning peripheral anterior-chamber-depth analyzer. Br J Ophthalmol 2004; 88: 1036-1041.
11. Sihota R, Lakshmaiah NC, Walia KB, et al. The trabecular meshwork in acute and chronic angle closure glaucoma. Ind J Ophthalmol 2001; 49: 255-259.
12. Alvarado JA, Murphy CG. Outflow obstruction in pigmentary and open-angle glaucoma. Arch Ophthalmol 1992; 110: 1769-1772.

