Awareness and Practice Patterns of Pediatricians Regarding Retinopathy of Prematurity: A Multicenter Study
Nikki Doreen Angbue Te, MD, Marie Joan Loy, MD, Carlos Emmanuel Chua, MD, Jose Melvin Jimenez, MD, Rachelle Anzures, MD
Retinopathy of prematurity (ROP), a disorder caused by abnormal vascularization of the developing retina of preterm, low birth weight or high risk infants, is now recognized as an important cause of childhood blindness. Globally, at least 50,000 children are blind from ROP. In the Philippines, it was reported by Gilbert that 8.4% of children in a blind school study were diagnosed as visually impaired or blind due to ROP.
According to the WHO 2010 data, the Philippines ranked 8 out of 184 countries for the number of preterm births with a preterm birth rate of 14.9%, majority of which are at-risk for ROP. The incidence of ROP varies in different parts of the world depending on their level of development. In the Philippines, there are no population-based studies on the exact prevalence and incidence of ROP. In a recent multicenter study, incidence reported was 13.8% among babies less than 36 weeks gestational age, 6.2% of which were between 33-36 weeks gestational age.
Recognizing this fact, the World Health Organization (WHO) Vision 2020 program has identified ROP as a leading cause of “avoidable disease” in the developing world requiring early detection and treatment to prevent blindness and the inherent costs to the individual and the community. An effective ROP screening program starts at the level of the pediatricians who are the first-liners in the identification of these infants at-risk for ROP. It is, therefore, essential to assess the pediatricians’ knowledge of the guidelines and practice patterns with regard to ROP screening. There is currently no local published study on the awareness and practice patterns of pediatricians on ROP.
This study determined the current knowledge of our pediatricians regarding ROP screening guidelines as recommended by the Philippine Pediatric Society (PPS), their referral practices, and barriers with regards to ROP screening. The study also measured the difference in referral rates among the different specialists (residents, general pediatricians, and subspecialists; neonatologists and non-neonatologists); years of practice; area of practice (city and province); type of practice (private and government); level of hospital (primary, secondary, and tertiary); and number of preterm babies seen.
Data gathered provided essential information to the ROP Working Group of the Philippine Academy of Ophthalmology (PAO) the current status of care of ROP patients in terms of screening and referral practices of the pediatricians and to help plan, formulate, and implement screening and awareness programs. The data enabled the group to set priorities to address the most common barriers and to identify the specific subgroups that need the most attention.
METHODOLOGY
This is a prospective, multicenter, cross- sectional study of pediatricians, both consultants and trainees. Inclusion criteria were as follows: hospital- based pediatricians from government or private institutions, practicing in the city or province, and signed informed consent. Exclusion criteria were as follows: pediatricians with no hospital affiliations, non-completion of the survey, and refusal to sign the consent. This study was approved by the Institutional Ethics Review Board of St. Luke’s Medical Center.
Outcome Measures
Primary outcome measures were the following: rate of awareness of the content of the Philippine Pediatric Society (PPS) ROP screening recommendations, rate of referral, criteria (timing, cut-off gestational age, and birth weight) used for referring and barriers encountered. Secondary outcome measures were the following: frequency of referral, guidelines followed, availability of a protocol, medical contraindications, ophthalmologist they refer to, difference in referral rates between the subgroups, and role of PAO to increase awareness of ROP.
Survey Instrument Development
A questionnaire was framed based on previously published knowledge, attitude, and practice pattern studies of pediatricians regarding ROP.8-10 Other questions were formulated after consulting with pediatricians, neonatologists, pediatric ophthalmologists, and retina specialists. The instrument was pilot tested on 30 consultants and 10 residents from a private tertiary institution. Feedback was obtained and modifications were made based on the effectiveness of the questions in soliciting the proper information, ease of comprehension, and relevance. Final instrument consisted of 26 questions, which were primarily multiple-choice and required less than 10 minutes to complete. An online survey was also created for the convenience of the respondents from remote areas.
Sample Size Determination
Sample size was determined using the assumption that 87% of the respondents were aware of ROP,9 with a maximum allowable error of 3% and a reliability of 90%; it was calculated to be 338. Assuming at least 60% response rate, the number to be recruited was 564.
Survey Administration
From July until September 2013, the questionnaire was disseminated to 600 pediatricians from 40 randomly chosen hospitals taken from the list of hospitals accredited by the Philippine Pediatric Society and Department of Health. Random sampling was achieved using the randomizer.org to generate random numbers. Dissemination was performed through the following ways: administered during monthly conferences in their respective hospitals, delivered to their respective clinics, and through email as in the case of online survey. Non-responders were followed-up to explain the purpose and relevance of the survey.
Data Analysis
Responses noted on completed questionnaires were complied with Excel and analyzed using SPSS ver. 17.0 and Stata software. The frequency distribution of each answer was noted in percentages. Chi square test was used to analyze the differences in referral rates among the different subgroups: physician classification (residents vs. general pediatricians vs. subspecialists, neonatologists vs. non-neonatologists); years of practice; area of practice (city vs. province); type of practice (private vs. government); level of hospital (primary vs. secondary vs. tertiary); number of preterm babies seen; availability of protocol, Neonatal Intensive Care Unit (NICU), and ophthal- mologist; and perceived success of timely ROP treatment. Availability of protocol and each of the barriers were analyzed by area of practice (city or province), type of practice (private or government), and level of hospital (primary, secondary, or tertiary). We considered a p < 0.05 to be statistically significant.
RESULTS
Response Rate and Demographic Characteristics
Out of the 600 who were given the survey, 10 directly declined to answer the survey and 172 were non-responders. A total of 418 respondents participated in the study. However, 7 surveys were noted to be incomplete and 2 failed to sign the consent form. They were immediately excluded from the study. We had a total of 409 eligible surveys. The response rate was 68.1%. Majority of the respondents were from Luzon (93%, 381/409), 2% (10/409) from Visayas, and 5% (18/409) from Mindanao. The respondents’ demographic and practice characteristics are presented in Table 1.
Knowledge of PPS ROP Screening Recommendations
Only 45% (182/409) were aware of the PPS recommendation on the timing of first eye examination for ROP screening, 49% (200/409) of the cut-off gestational age, and 42% (173/409) of the cut-off birth weight recommended by the PPS for referring at-risk babies with stable clinical course. Overall, considering the timing, cut-off gestational age, and cut-off birth weight together, most respondents (81%, 333/409) reported them incorrectly.
ROP Screening Practices
Nearly all (92%, 378/409) respondents referred their at-risk preterm babies to an ophthalmologist for ROP screening. Among those who referred (n=378), the frequencies of referral were as follows: 61% always referred, 24% often referred, 10% and 4% referred sometimes and rarely respectively. Among the 8% (31/409) who did not refer, reasons for not referring to an ophthalmologist were as follows: they referred all their preterm babies to neonatologists, leaving the decision to them whether or not to refer; and failure of at-risk preterm babies to reach the recommended timing for referral as were the cases in 2 tertiary government hospitals.
Among those who referred, 60% (225/378) reported that they did not abide by any specific ROP screening guidelines. Of the 40% (153/378) who stated that they followed certain guidelines, only 83 practitioners indicated the guidelines they followed: 36% (30/83) followed the American Association of Pediatrics (AAP) ROP screening guidelines, 34% (41/83) the PPS recommendation, 15% (18/83) used either the AAP or PPS, and 4% (5/83) used the guidelines recommended by the Ophthalmology Department of their institution.
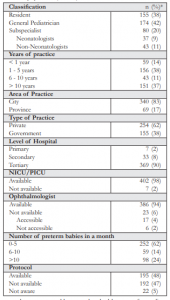
Protocol on ROP screening was available in 48% (195/409), not available in 47% (192/409), and 5% (22/409) were not aware of the availability. Tertiary institutions were more likely to have an ROP screening protocol compared to secondary and primary institutions (52% vs. 12% vs. 14%, p < 0.001). Respondents practicing in the city were more likely to have a protocol compared to provincial practice (52% vs. 25%, p< 0.001). There was no significant difference in the availability of a protocol in private and government institutions (p=0.09).
Respondents whose institutions had protocols on ROP screening were more likely to refer (97% vs. 88%, p=0.002). Respondents from tertiary institutions were more likely to refer compared to those from secondary and primary institutions (94% vs. 82% vs. 86%, p=0.042). Similarly, respondents from private institutions were more likely to refer compared to government institutions (95% vs. 88%, p=0.016). Neonatologists (p=0.131), longer years of practice (p=0.55), more preterm babies seen (p=0.786), availability of NICU (p=0.499), ophthalmologist (p=0.547), and city practice (p=0.167) were not significantly correlated with better referral rates. Table 2 lists the referral rates in relation to the demographics and practice profile.
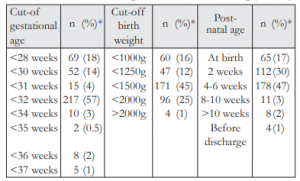
In their private practice, more than half (57%, 217/378) of those who referred used the currently recommended criteria of 32 weeks or less, while 45% (171/378) used the currently recommended criteria of 1500g or less. Only 47% (178/378) used the recommended criteria of 4-6 weeks post-natal age for referring at-risk babies for ROP screening. Table 3 lists the different criteria and timing used by the respondents for referring their at-risk preterm babies for ROP screening.
About 46% (186/409) referred premature babies for ROP screening irregardless of any medical condition, while 39% (161/409) considered intubation as a medical contraindication to referring. Other medical contraindications considered were: active infection (24%, 99/409), very low birth weight (11%, 46/409), and apnea or unstable clinical course (2%, 8/409).
Almost half (47%, 194/409) referred their babies exclusively to a pediatric ophthalmologist for ROP screening, while 12% (48/409) and 14% (56/409) referred exclusively to a retina specialist and a general ophthalmologist, respectively. Only 27% (111/409) referred to any ophthalmologist depending on availability.
The inability of families to follow-up after discharge was the most commonly encountered barrier to ROP screening (41%, 167/409). This was followed by high expense of the screening (38%, 154/409), unavailability of ophthalmologists (16%, 64/409), and safety concerns of the parents (13%, 51/409). About 33% (135/409) reported not encountering any barrier to referring. Poor follow- up by the parents was more likely in government compared to private institutions (60% vs. 29%, p < 0.001). The lack of ophthalmologists (28% vs. 8%, p < 0.001) and safety concerns (21% vs. 8%, p < 0.001) were also more likely in government compared to private institutions. Among the previously mentioned barriers [poor follow-up (p=0.753), lack of ophthalmologist (p=0.168), and safety concerns (p=0.522)], no significant difference was found between city and provincial practice. High expense as a barrier was more likely to be encountered in the province compared to the city (52% vs. 35%, p=0.006). There was no significant difference on reports of high expense as a barrier in private and government institutions (p=0.07). Absence of any barrier to ROP screening was more likely encountered in the city compared to the province (35% vs. 22%, p=0.029). Similarly, reports of the absence of any barrier were more likely encountered in private compared to government institutions (44% vs. 15%, p < 0.001). There was no significant difference in each of the following barriers encountered [poor follow-up (p=0.593), high expense (p=0.338), safety concerns (p=0.599), and absence of barrier (p=0.665)] between primary, secondary, and tertiary institutions. However, the absence of an ophthalmologist as a barrier was more likely in a primary institution compared to a secondary or tertiary institution (57% vs. 9% vs. 15%, p=0.006).

When the respondents were asked on ways to increase awareness of ROP, 69% (281/409) stated that conducting lectures and workshops to pediatricians is the best way to improve awareness of ROP. This was followed by distribution of educational materials at 7% (28/409) and conducting lay fora at 3% (14/409). One respondent reported that PAO should educate their own members since not all ophthalmologists are adept at ROP screening. About 20% (85/409) reported that PAO should employ multiple strategies to improve awareness of ROP.
DISCUSSION
Owing to the preventable nature of ROP early in its course, a good screening protocol is pivotal. The goal of screening is to detect serious ROP that is within the window of opportunity for the optimal application of proven therapy. For the pediatricians, ROP screening recommendations should guide them on who are at-risk and should be referred and when to refer appropriately for the initial eye examination. Table 4 lists the Philippine Pediatric Society ROP screening guideline as adopted from the American Academy of Pediatrics (AAP) published in 2006, and recommended by the Philippine Academy of Ophthalmology, Philippine Society of Pediatric Ophthalmology and Strabismus, and Vitreoretinal Society of the Philippines: this was the guideline followed by majority of our respondents.
In our study, we found that majority were unaware of the criteria of the PPS ROP screening recommendations when considering the timing, gestational age, and birth weight all together. Less than half (45%) were aware of the recommended timing of 4-6 weeks and a marginally similar proportion (47%) used this timing. This was relatively low compared to the study by Rani in India which showed that 71.1% were aware of the timing of the first eye examination.9 A similar proportion (47%) also referred earlier than usual which was not in accordance with the standard of care. However, this might represent a more cautious approach in order to avoid missing any predisposed babies.
Among the respondents, almost half (49%) were aware of the cut-off gestational age recommended by the PPS for referring predisposed babies for ROP screening and more than half (57%) used this recommended criterion of 32 weeks or less. However, more respondents favored the use of a lower, more limiting criterion (36%) compared to a higher, more comprehensive criterion (7%). This is in contrast to the study by Rani and Kemper where majority of the respondents used a higher, more comprehensive criterion to avoid missing any ROP cases.9,11 A slightly lesser number (42%) were aware of the cut-off birth weight recommended by the PPS and comparable proportion (45%) used this recommended criteria of 1500g or less. More respondents used a lower, more limiting criterion (28%) compared to a higher, more comprehensive criterion (26%), similar to previously mentioned studies.

The criteria followed by the pediatricians in this study were highly variable. Those who considered the lower, more limiting criteria might have a higher risk of missing out babies. Alternatively, following the higher, more comprehensive criteria for gestational age and birth weight did not necessarily equate to poor practice. This might represent a more defensive approach to avoid missing babies who might develop ROP. Another situation that we should consider was that in Eastern and developing countries like ours, there is a rise in incidence of ROP, with severe ROP more likely to be seen in babies with higher gestational age and birth weights. This was observed in Vietnam, China, India, Iran, Saudi Arabia, and Turkey.12-17 Hence, the recommendations were to use a wider screening criteria for identifying predisposed babies,18 to evaluate prospectively and not to rely blindly on the criteria published by developed countries.
In our study, only 8% did not refer. This was in contrast to the study by Padwardhan where 34% of pediatricians did not refer at all and a significantly higher proportion were rural practitioners.10 Their government physicians had a higher rate of non- referral compared to private practitioners. In our study, there was no significant difference in rates of non-referral in the city and province. Our pediatricians practicing in government institutions had a higher rate of non-referral. This could be explained by the higher reports of poor follow-up by the parents after discharge and non-availability of ophthalmologists in government hospitals. In our study, poor follow-up after discharge was the most commonly encountered barrier to ROP screening (41%); followed by high expense (38%), un- availability of ophthalmologists (16%), and safety concerns of the parents (13%). Kemper reported the lack of available ophthalmologists (26%) as the major barrier and the inability of parents to follow- up after discharge (83%) as the overall barrier.11 Padwardhan stated that the non-availability (23%) and inaccessibility (41%) of trained ophthalmologists as the major barriers encountered among those who did not refer,10 while Rani reported on the unwillingness of parents to ROP screening (18.4%), unawareness of referral facilities (15.8%), and high expense (13.1%).9 Our study also found that safety concerns as a barrier were more likely encountered in government compared to private institutions, possibly due to the parents’ lack of knowledge about ROP or the inability of pediatricians in those institutions to explain the screening procedure and implications of failure to do ROP screening for predisposed babies.
To date, there are no published medical contraindications to ROP screening. A major misconception among the respondents was that intubated babies can not be examined. Although there have been reports that the dilating drops used may have some systemic effects on the baby, the decision whether or not to screen lies on the discretion of the neonatologist and the ophthalmologist.
The strength of this study lies in the extensive distribution of the survey and the relatively large number of respondents who completed the survey. Due to the limited time and resources, however, the data gathered from the Visayas and Mindanao areas may not be representative of the population in those areas. A focused and individualized approach may yield a better response rate. As a survey, the response was voluntary; refusal to answer the survey was always a possibility, including the fact that respondents might have responded in a manner they felt they should respond and not in accordance with actual practice (social desirability bias). Recommendations for further studies include assessing the neonatology and ophthalmology workforces, and to conduct a regional survey to better assess which region needs the most attention.
In summary, majority of the pediatricians surveyed referred their at-risk babies for ROP screening, even if only a few were aware of the PPS ROP screening recommendations. Many did not have an established protocol for ROP screening, leading to varied practice patterns of pediatricians with regards to ROP screening. Barriers to screening were mostly encountered in government and primary hospitals.
Given the current situation, there is still a need to increase awareness among the pediatricians. The PPS and PAO, together with the Department of Health (DOH) should have a consensus and uniform national published ROP screening guideline, taking into account the current incidence of ROP in older and larger babies. They should be the prime movers to ensure that ROP awareness and screening guidelines are disseminated uniformly among their members. We recommend strict implementation of a written protocol on the prevention, screening, treatment, and follow-up of ROP in all hospitals involved in newborn delivery, since this was correlated with significantly better referral. Establishment of a referral system and open communication between neonatologists, pediatricians, obstetricians, ophthalmologists, and especially the parents regarding the ROP screening plans to ensure that appropriate eye care needs are delivered. The scarcity of qualified ophthalmologists in some rural and government areas may be remedied by the advent of tele-ophthalmology, conduction of training and workshops for ophthalmologists, and establishment of referral facilities. The impact of an effective ROP screening program on blindness prevention is huge and must be addressed.
ACKNOWLEDGMENT
Sincere gratitude is extended to the following who helped make this paper possible: Kristine Corpus MD, Perlen Chua MD, Irminia Ang MD, and Karen Lim MD for the unwavering guidance in developing the questionnaire; Lambert Ilagan MD, Victoria Wee Eng Binas MD, Rachelle Anzures MD, Nathaniel Paul Chan MD, and John Colacion MD for helping disseminate the survey; Macario Reandelar MD MSPH for the statistical analyses; Noel Atienza MD and Joseph Anthony Tumbocon MD for sharing their insights. Lastly, to the proponent’s family for the unwavering moral and financial support.
REFERENCES
1. Policy Statement. Screening Examination of Premature Infants for Retinopathy of Prematurity. Section on
Ophthalmology, American Academy of Pediatrics, American Academy of Ophthalmology, and American Association for Pediatric Ophthalmology and Strabismus. Pediatrics 2006;117:572-76.
2. Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk, and implications for control. Early Hum Dev 2008;84:77-82.
3. Gilbert C, Fielder A, Gordillo L ,et al. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics 2005;115;518-25.
4. Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends for selected countries since 1990: a systematic analysis and implications. World Health Organization, 2012.
5. Corpus K, Jimenez JM, Anzures R, et al, ROP Working Group. Proposed new retinopathy of prematurity screening criteria: evidence for including older and heavier Filipino premature babies. Philipp J Ophthalmol 2013;38:72-79.
6. Gilbert C, Foster A. Childhood blindness in the context of VISION 2020-the right to sight. Bull World Health Organ 2001;79:227-32.
7. Hernandez E. Retinopathy of Prematurity. Standards of Newborn Care. 3rd ed. Philippines: Philippine Society of Newborn Medicine, Subspecialty of Philippine Pediatric Society. 2008;135-6.
8. Bhattad K, Patani A. Knowledge, attitude, and practice patterns of retinopathy of prematurity among ophthalmologist and pediatrician. Community/Social Ophthalmol 2012;626-30.
9. Rani PK, Jalali S. Knowledge, attitude, practice study of retinopathy of prematurity amongst pediatricians attending a neonatal ventilation workshop in South India. World J Retina & Vitr 2011;1:9-13.
10.Patwardhan SD, Azad R, Gogia V, et al. Prevailing clinical practices regarding screening for retinopathy of prematurity among pediatricians in India: A pilot survey. Indian J Ophthalmol 2011;59:427-30.
11.Kemper AR, Wallace DK. Neonatologists’ practices and experiences in arranging retinopathy of prematurity screening services. Pediatrics 2007;120:527-31.
12.Phan MH, Nguyen PN, Reynolds JD. Incidence and severity of retinopathy of prematurity in Vietnam, a developing middle-income country. J Pediatr Ophthalmol Strabismus 2003;40:208-12.
13.Chen Y, Li X. Characteristics of severe retinopathy of prematurity patients in China: A repeat of the first epidemic? Br J Ophthalmol 2006;90:268-71.
14.Deshpande DA, Chaturvedi M, Gopal L, et al. Treatment of threshold retinopathy of prematurity. Indian J Ophthalmol 1998;46:15-9.
15.Karkhaneh R, Mousavi SZ, Riazi-Esfahani M, et al. Incidence and risk factors of retinopathy of prematurity
in a tertiary eye hospital in Tehran. Br J Ophthalmol 2008; 92:1446-9.
16.Binkhathlan AA, Almahmoud LA, Saleh MJ, Srungeri S. Retinopathy of prematurity in Saudi Arabia: Incidence, risk factors, and the applicability of current screening criteria. Br J Ophthalmol 2008;92:167-9.
17.Basmak H, Niyaz L, Sahin A, et al. Retinopathy of prematurity: Screening guidelines need to be reevaluated
for developing countries. Eur J Ophthalmol 2010;20:752-5.
18.Shah PK, Narendran V, Kalpana N, Gilbert C. Severe retinopathy of prematurity in big babies: History repeating itself? Indian J Pediatr 2009;76:801-4.

