A Randomized Controlled Trial on the Effect of Lutein Supplementation on Macular Pigment Optical Density and Macular Function in Pseudophakic Patients
Victor Ephraime V. Paulino, MD, DPBO, Edward C. Uy, MD, DPBO,
Sherman O. Valero, MD, FPAO
Makati Medical Center, Department of Ophthalmology, Makati City, Philippines
Correspondence: Victor Ephraime V. Paulino, MD, DPBO
Department of Ophthalmology, Makati Medical Center
#2 Amorsolo Street, Legaspi Village, Makati City, Philippines
e-mail: rempaulino@gmail.com
Disclaimer: The authors declare no conflict of interest.
The human eye contains pigments concentrated at the center of the retina or macula lutea that are believed to protect the retinal pigment epithelium (RPE) and photoreceptors.1-3 Collectively known as macular pigments, the three isomeric hydroxyl carotenoids: lutein, zeaxanthin, and meso-zeaxanthin, are considered key components of the retina’s internal defense system against phototoxicity.4-7 These carotenoids absorb short-wavelength blue light, act as a filter that limits photochemical damage, and as antioxidants shown to protect against light-induced oxidative damage in the retina.
Age-related macular degeneration (AMD) is a progressive disease of the macula and the primary cause of visual and functional impairment in the developed world.8,9 While continuous research on the treatment of AMD continues, there is also substantial interest in preventing or reducing the progression to AMD through interventions on modifiable risk factors. Dietary changes and dietary supplementation are particularly appealing due to their universal applicability and relatively low cost.4,10 The Age-Related Eye Disease Study (AREDS) demonstrated that daily oral supplementation with antioxidant vitamins and minerals reduced the 5-year risk of advanced AMD by 25%.11 The AREDS2 then looked at changing the original formula, specifically the addition of lutein/zeaxanthin (10 mg/2 mg), omega-3 fatty acids (docosahexaenoic acid/eicosapentaenoic acid [350 mg/650 mg]), or both, and showed no apparent effect of β-carotene elimination on progression to advanced AMD.12
Some studies on macular pigments optical density (MPOD) have demonstrated that the concentration of macular pigments in patients with AMD is significantly lower than in normal, healthy eyes, while others have shown no differences in MPOD between normal eyes and those with varying stages of AMD.13-15 Additionally, lower carotenoid levels may be a risk factor for AMD progression.15,16 Several studies including the Carotenoids in Age-Related Eye Disease Study (CAREDS), the Blue Mountain Eye study, and AREDS2 established that diets low in lutein/ zeaxanthin increased the risk of AMD, although CAREDS did not find a consistent association between MPOD and AMD.13,17,18 Therefore, the assessment of factors influencing MPOD is important.
The natural crystalline lens also provides much needed protection to the eye by absorbing light rays within the 300-400 nm wavelengths, further augmented with age-related yellowing of the lens with advanced age, which then may progress to clinically significant cataract.19-21 Removal of the cataract and replacement by a standard intraocular lens (IOL) subsequently increase the exposure of the retina to blue light. Given that higher exposure to harmful light decreases MPOD in postoperative eyes, a decrease in MPOD is anticipated over time.16,19 The current standard of care in the immediate post-operative period after cataract surgery includes topical antibiotics and topical corticosteroids. Anti-oxidant supplementation is not typically recommended, unless warranted for a preexisting or newly diagnosed ophthalmic condition.
The objective of this randomized study was to prospectively evaluate the efficacy of oral lutein supplementation on MPOD levels and macular function and recovery in pseudophakic eyes that underwent phacoemulsification.
PATIENTS AND METHODS
This prospective, randomized, parallel-arm, single-masked study was conducted at the Makati Medical Center, in Makati City, Philippines, and was approved by Makati Medical Center Institutional Review Board. The study was conducted in accordance with the tenets of the Declaration of Helsinki.
Written informed consent was obtained, either in English and Filipino, from patients who had undergone phacoemulsification surgery with IOL implantation not earlier than 3 months and had not received lutein supplementation within the previous 6 months. Patients were excluded if they had a history of chloroquine, hydroxychloroquine, ethambutol, or tamoxifen use; significant media opacity (corneal scar, posterior capsular opacity, vitreous hemorrhage); maximum pupil diameter greater than 6 mm; missing fixation; retinal pathology such as severe non-proliferative to proliferative diabetic retinopathy, category 4 AMD, vitreoretinal interface syndrome, myopic macular degeneration, vascular occlusion, or retinitis pigmentosa; other ocular diseases, including glaucoma, uveitis, strabismus, or optic nerve disease; or history of retina-vitreous lasers or surgeries in the study eye.
Following study screening procedures, patients were enrolled and randomized 1:1 to receive active treatment (lutein supplementation) or no treatment. Randomization was done using a computer-generated list of random numbers. Patients receiving lutein supplementation were classified under Group A, and those not receiving any treatment under Group B. Group A was dispensed with Lutax 20 (Santen Pharmaceutical Co. Ltd, Japan) which contains 20 mg lutein, 0.1 to 0.6 mg sodium, 0.03 g carbohydrate, 0.20 g fat, 0.09 g protein, gelatin, safflower oil, marigold pigment, glycerin, and bees wax. Patients were advised to take one tablet daily, each morning after breakfast, and to store the container of unconsumed tablets according to the product label. Study supplements were provided to patients every 4 weeks starting at day 0 until week 8, and pill-counting and medication compliance were assessed from week 4 until week 12. Failure to take the supplement for more than 3 consecutive days within the 12-week supplementation period was labeled as non-compliance, and noncompliant patients were discontinued from the study.
Further subgroup analyses were done based on IOL type, i.e., Group A1 (with supplementation, clear IOL), Group A2 (with supplementation, yellow IOL), Group B1 (no supplementation, clear IOL), and Group B2 (no supplementation, yellow IOL).
The investigation period was for 16 weeks, which included 12 weeks of daily supplementation for Group A, beginning after the completion of baseline assessments on day 0. Patients were assessed at 4- week intervals (day 0, weeks 4, 8, and 12) and were followed for an additional 4 weeks after discontinuing supplementation to week 16. Study assessments included uncorrected distance visual acuity, bestcorrected visual acuity (BCVA), and low-luminance visual acuity (LLVA) using Early Treatment Diabetic Retinopathy Study (ETDRS) charts, manifest refraction, slit-lamp biomicroscopy, assessment of pupillary reflexes and size, intraocular pressure (IOP) measurement by Goldmann applanation tonometer, MPOD, photostress test (PST), dilated fundus examination, and recording of adverse events based on patient reports and clinical examination.
MPOD was measured using heterochromatic flicker photometry (HFP) with a Macular Pigment Screener MPS II (Elektron eye technology, Cambridge, United Kingdom) at 0.5° of retinal eccentricity using standard mode. The MPOD can be calculated (results on a scale of 0 to 1) through this method by measuring the absorbance of blue light by the MP. For lower values, the level of blue light that reaches the macula is higher. Further details of this method are described previously by van der Veen et al. 20 and Howells et al.21 Patients underwent three repeated assessments for each part of the test, and the examination was deemed acceptable if the standard error in the mean MPOD was less than 0.020 absorbance units (AU).
PST is a dynamic test of macular performance based on precisely measuring the time required for a patient to recover sufficient visual function to perform a defined visual task after he has been dazzled with an intense flash of light (i.e., visual recovery time or VRT)22. Following BCVA assessment, while the fellow eye is occluded, the study eye was subjected to a bright light from an ophthalmoscope held 2 to 3 cm from the eye and directed onto the macula for 10 seconds. The subject was then asked to read the line of letters just above his/her best line of VA. VRT is defined as the time interval from when the ophthalmoscope was removed to the time when the defined visual task was completed.
Treatment assignment was masked to study assessors of VA, MPOD, and PST, and to the statisticians. The primary investigator, patient, and a study personnel tasked to dispense the study medications were aware of the patient’s treatment group.
Statistical analysis
Descriptive statistics were used to summarize the clinical characteristics of the patients. Frequency and proportion were used for nominal variables, median and range for ordinal variables, and mean and SD for interval/ratio variables. Independent T-test was used to determine the difference of mean, median and frequency between groups.
The primary outcome of the study was the difference in the mean MPOD between the two groups during the different timepoints of the study. The secondary outcomes were the between-group differences in BCVA, LLD, and PST. MPOD, BCVA, LLD, and PST were all analyzed using a two-way analysis of variance (ANOVA) using SPSS 20. The difference in parameters from baseline to week 16 was assessed, as well as whether a significant difference was observed between treatment groups. All correlation analysis was carried out using Pearson correlation in SPSS 20. A p-value <0.05 was considered significant.
The rate of MPOD change per 4 weeks of supplementation was computed using the formula: {[(µMPOD4 – µMPOD0) + (µMPOD8 – µMPOD4) + (µMPOD12 – µMPOD8)] / 3}*100; where “µMPOD(x)” is the mean (µ) MPOD measured per week interval (x: weeks 0, 4, 8, and 12) respectively. Likewise, LLD was computed by getting the difference between LLVA and BCVA in corresponding LogMAR values: LLD = LLVA – BCVA.
A subset analysis of collected data was made to determine if the type of IOL implanted (clear versus yellow IOL) had an effect on the MPOD and macular function.
RESULTS
One hundred twenty-eight (128) pseudophakic eyes were randomized, 64 eyes in each group and all completed the 16-week study period. Group A1 included 33 eyes, Group A2 included 31 eyes, Group B1 included 29 eyes, and Group B2 included 35 eyes.
At baseline, the mean MPOD level of all study eyes was 0.35 ± 0.18 density units (DU). The MPOD levels by gender and age groups are shown in Table 1.
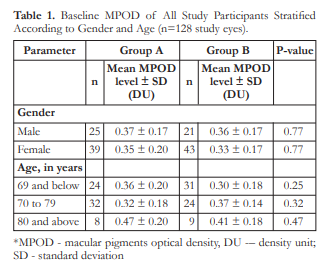
Figure 1 shows the change in MPOD from baseline to week 16. The means of MPOD of Groups A and B were similar at baseline (p=0.326). Beginning at week 4 and through week 16, significant differences in the means of MPOD were observed between the 2 groups. At each timepoint from week 4 to week 16, group A had a significantly higher mean MPOD than group B (Figure 1). Furthermore, from baseline to week 12, a significant mean increase of 6.32 ± 1.72% per 4 weeks of supplementation was observed in the mean MPOD of Group A. The mean MPOD increased by 4.98% after 4 weeks of supplementation and was highest from weeks 8 to 12 with a mean MPOD increase of 8.27%. The mean MPOD level at week 12 was 0.55 ± 0.14 DU which was sustained at 0.54 ± 0.15 DU at week 16. In Group B, MOPD decreased by a mean rate of 0.63 ± 0.48% from week 0 to week 12 and a cumulative decrease of 1.56% was noted from baseline to 16 weeks (0.34 ± 0.17 to 0.33 ± 0.16 DU, respectively).

Figure 2 shows the change in BCVA from baseline to week 16 between treatment groups (p=0.390). For Group A, the means of BCVA at baseline and week 16 were LogMAR 0.047 ± 0.049 and 0.030 ± 0.032, respectively. For Group B, they were LogMAR 0.036 ± 0.045 and 0.036 ± 0.036, respectively. Furthermore, independent sample t-test was carried out at different timepoints between the two groups which showed no significant difference except at weeks 8 (p=0.022) and 12 (p=0.006), wherein Group B had a higher mean VA than Group A.

Figure 3 shows the change in the means of LLD over time. Both groups had similar LLD at baseline (p=0.549). Significant differences in LLD was observed beginning week 8 with Group A demonstrating lower mean LLD than group B at weeks 8, 12, and 16. Furthermore, after 12 weeks of supplementation, Group A demonstrated significant improvement in mean LLD (p=0.017) from baseline.
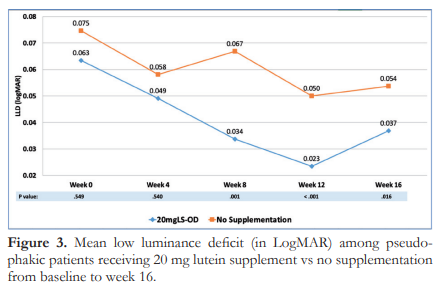
Figure 4 shows the change in the means of VRT over time. Similar to LLD, the initial 4 weeks of supplementation did not differ between groups, however, significant diference was noted starting week 8 (p<0.001). Additionally, Group A had significant improvement in VRT from 83.06 at baseline to 68.80 seconds at 12 weeks was noted (p=0.000). This improvement in macular function was sustained after discontinuing supplementation at week 12, with a mean VRT of 67.45 ± 15.84 seconds at week 16. In contrast, Group B showed no change in mean VRT over time (85.03 ± 28.04 seconds to 85.14 ± 16.58 seconds at weeks 0 and 16, respectively, p=0.616) .
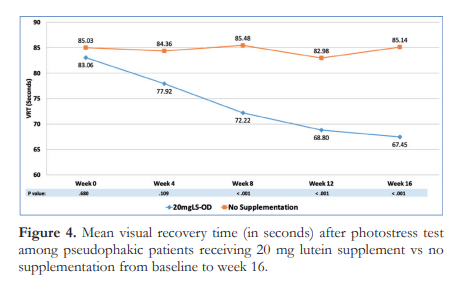
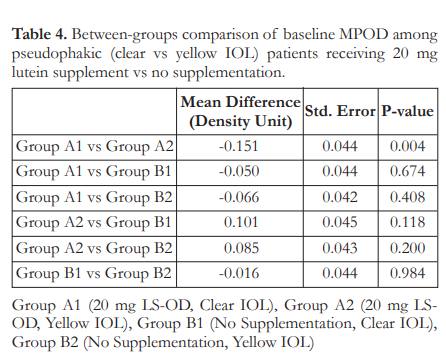
A subgroup analysis based on IOL color showed that the mean MPOD levels of Group A1, Group A2, Group B1, and Group B2 were significantly different from each other at all study time points (p<0.001) (Figure 5).
Figure 5 further shows that in Group A1, a significant rise in MPOD was observed from week 0 to week 12 with a mean increase of 7.24 ± 2.56% per week of supplementation. A similar observation was also noted in Group A2, with a mean MPOD increase of 5.33 ± 1.88% per week of supplementation. In both lutein-supplemented groups, the MPOD level was sustained until 16 weeks at 0.49 DU for Group A1, and 0.59 DU for Group A2.
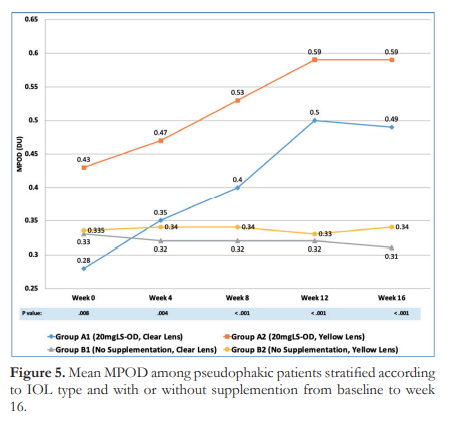
On the other hand, an average MPOD decrease of 0.49 ± 0.33% was observed from week 0 to week 12 for Group B1 while Group B2 showed a mean MPOD decline of 0.74 ± 0.63% from week 0 to week 12. This is consistent with the general trend for the non-supplemented group.
Cumulatively, a decrease of 2.07 and 1.14% for Groups B1 and B2, respectively, were noted after 16 weeks of observation. At this timepoint, both notreatment subgroups showed a significantly lower MPOD level compared with the lutein-supplemented subgroups while not being significantly different from each other.
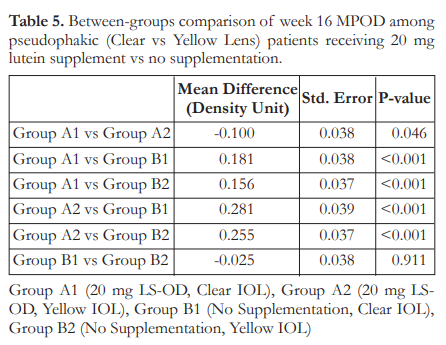
Tukey’s post-hoc analysis of baseline mean MPOD was carried out for pairwise comparisons among the four subgroups. At baseline, only Groups A1 and A2, with mean MPOD of 0.28 and 0.43 DU respectively, showed signficanct difference; with Group A2 having the highest mean MPOD (Table 4). At week 16, all comparisons showed a significant difference (Table 5) except those between Group B1 and Group B2 (p=0.911).
Finally, participants of this study did not report any adverse effects nor observed physical changes during the entire 16-week study period. All members of Group A were compliant with the supplementation and its regimen; and participants were able to tolerate all the assessments performed.
DISCUSSION
This randomized controlled trial investigating the effects of an oral lutein supplement on MPOD and macular function provides the first clinical data among Filipino pseudophakic patients. The study showed a baseline MPOD level of 0.35 ± 0.18 DU that is consistent with previous local studies involving healthy, un-operated, Filipino eyes.23,24 Gender and age did not appear to be associated with MPOD level. However, a link between reduced MPOD and presence of AMD has been suggested by several trials.15,25
With sufficient magnitude, almost all portions of the electromagnetic (EM) spectrum can cause damage to the eye. The most offending portions of the EM spectrum are the UV-A (315 nm to 400 nm), UV-B (280 nm to 315 nm), and “blue-light” portion of the visible spectrum (380 nm to 500 nm). The retina is most sensitive to light at the shorter wavelengths (maximum sensitivity shown at 441 nm) and retinal damage at the shorter visible wavelengths (up to 500 nm) is primarily photochemical in nature. In the Chesapeake Bay Watermen Study, late AMD was positively correlated to cumulative sunlight exposure; blue light (380 to 500 nm) showed the greatest effects, possibly due to photochemical or photo-oxidative damage in the RPE. 26
While it has long been hypothesized that exposure to UV light and undergoing cataract surgery could be independent risk factors for the development and progression of AMD, the results from clinical follow-up studies with patients implanted with bluelight-filtering/yellow IOLs have not directly confirmed their protective impact to the macula and the prevention of macular degeneration in humans.27-29 This current study demonstrated that lutein supplementation promotes increasing MPOD, regardless of IOL type.
The 20-mg/tablet lutein supplement taken once daily was shown to be an effective and safe in raising MPOD levels in pseudophakic eyes. This can, theoretically, confer valuable protection to the macula against the harmful effects of blue light.30 Lutein supplementation resulted to a significant increase in MPOD levels starting at 4 weeks, which persisted during the entire 12 weeks of supplementation. The effect was sustained up to week 16 or 4 weeks after discontinuation of the study medication. In addition, the 20 mg lutein supplement resulted in a significant rise in MPOD levels compared with those not receiving supplementation. Dietary supplementation with lutein and its positive effects on MPOD and protection against the progression of AMD have also been demonstrated previously in other population studies.10,31
The rise in MPOD level following lutein supplementation did not substantially contribute to improving BCVA specially among individuals who had satisfactory baseline vision. At LogMAR 0.04 ± 0.04, the margin for improvement is narrow and the effect of lutein on visual acuity may be less pronounced compared with changes expected on diseased eyes, as shown previously with AMD patients32.
Studies have supported VRT and LLVA as sensitive functional markers of the macula, especially in early cases of AMD33,34. Lutein supplementation at 20 mg per day resulted in a significant reduction in LLD starting at 8 weeks of supplementation and varied greatly from those not receiving supplementation. Dietary supplementation can positively benefit visual function and decrease the chances of subsequent visual acuity loss, especially in diseased eyes.
Our study shows that lutein supplementation significantly reduced VRT beginning at week 8 and sustained up to week 16. Of note, both groups showed higher than normal VRT values across all time points which can be attributed to the use of brighter and whiter LED bulbs during photostress test. In addition, removal of the crystalline lens, a natural filter of the eye, may dazzle patients for longer period of time.
It should be noted that when the supplementation and non-supplementation groups were subdivided further by the type of IOL (yellow versus clear), the four groups differed from one another, both at week 0 and at the end of the study. However, significance was seen only between Group A1 (lutein supplemented, clear IOL) and Group A2 (lutein supplemented, yellow IOL). The yellow IOL could be superior in conferring protection to the macula by providing the first degree of barrier and filtering blue light that may exhaust macular pigments. In this study, the yellow IOL had inherent protection to blue light and the lutein supplementation enhanced it further by increasing MPOD and macular function. Overall, it can be concluded that while yellow IOL provide macular protection, the addition of 20 mg lutein supplement once daily exhibits linear, direct, and high-level MPOD elevation that plateaus when supplementation is discontinued.
This randomized controlled trial demonstrated the benefits of oral 20 mg/tablet lutein supplement (Lutax 20) on MPOD and macular function among normal patients who underwent cataract surgery. Lutein supplementation can augment the needed protection of clear IOLs, to match or even exceed the basic protection offered by blue-light filtering IOLs.
These outcomes may improve the current standards of care for pseudophakic individuals, which aim to delay progressive visual and functional impairment.
REFERENCES
- Lima VC, Rosen RB, Farah M. Macular pigment in retinal health and disease. Int J Retina Vitrous 2016;2(1):19.
- Datta S, Cano M, Ebrahimi K, et al. The impact of oxidative stress and inflammation on RPE degeneration in nonneovascular AMD. Prog Retin Eye Res 2017;60:201-218.
- Handa JT, Cano M, Wang L, et al. Lipids, oxidized lipids, oxidation-specific epitopes, and Age-related Macular Degeneration. Biochim Biophys Acta Mol Cell Biol Lipids 2017;1862(4):430-440.
- Bernstein PS, Li B, Vachali PP, et al. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res 2016;50:34-66.
- Scripsema NK, Hu DN, Rosen RB. Lutein, zeaxanthin, and meso-zeaxanthin in the clinical management of eye disease. J Ophthalmol 2015;2015:865179. doi: 10.1155/2015/865179.
- Johnson EJ. A Biological Role of Lutein. Food Rev Int 2004;20(1):1-16.
- Yu M, Yan W, Beight B. Lutein and zeaxanthin isomers protect against light-induced retinopathy via decreasing oxidative and endoplasmic reticulum stress in BALB/cJ mice. Nutrients 2018;10(7):842.
- Jonas JB, Cheung CMG, Panda-Jonas S. Updates on the Epidemiology of Age-Related Macular Degeneration. Asia Pac J Ophthalmol (Phila) 2017:6(6):493-497.
- Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet 2018;392(10153):1147-1159.
- Eisenhauer B, Natoli S, Liew G, Flood VM. Lutein and zeaxanthin-food sources, bioavailability and dietary variety in age-related macular degeneration protection. Nutrients 2017;9(2):120.
- Age-Related Eye Disease Study Research Group. Risk Factors Associated with Age-Related Macular Degeneration – A Casecontrol Study in the Age-Related Eye Disease Study: AgeRelated Eye Disease Study Report Number 3. Ophthalmology 2000;107(12):2224-2232.
- Age-Related Eye Disease Study Research Group. Lutein + Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration: The Age-Related Eye Disease Study 2 (AREDS2) Randomized Clinical Trial. JAMA 2013;309(19):2005-2015.
- Berendschot TT, Willemse-Assink JJ, Bastiaanse M, et al. Macular pigment and melanin in age-related maculopathy in a general population. Invest Ophthalmol Vis Sci 2002;43(6):1928-32.
- Obana A, Hiramitsu T, Gohto Y, et al. Macular carotenoid levels of normal subjects and age-related maculopathy patients in a Japanese population. Ophthalmology 2008;115(1):147- 157.
- Arunkumar R, Calvo CM, Conrady CD, Bernstein PS. What do we know about the macular pigment in AMD: the past, the present, and the future. Eye 2018;32(5):992-1004.
- Obana A, Tanito M, Gohto Y, et al. Macular pigment changes in pseudophakic eyes quantified with resonance Raman spectroscopy. Ophthalmology 2011;118(9):1852-1858.
- Mainster MA. Violet and blue light blocking intraocular lenses: photoprotection versus photoreception. Br J Ophthalmol 2006;90(6):784-792.
- Mellerio J. Yellowing of the human lens: nuclear and cortical contributions. Vision Res 1987;27(9):1581-1587.
- Pescosolido N, Barbato A, Giannotti R, et al. Age-related changes in the kinetics of human lenses: prevention of the cataract. Int J Ophthalmol 2016;9(10):1506-1517.
- van der Veen RL, Berendschot TT, Hendrikse F, et al. A new desktop instrument for measuring macular pigment optical density based on a novel technique for setting flicker thresholds. Ophthalmic Physiol Opt 2009;29(2):127-317.
- Howells O, Eperjesi F, Bartlett H. Measuring macular pigment optical density in vivo: a review of techniques. Graefes Arch Clin Exp Ophthalmol 2011;249(3):315-347.
- Glaser JS, Savino PJ, Sumers KD, et al. The photostress recovery test in the clinical assessment of visual function. Am J Ophthalmol 1977;83(2):255-260.
- Santos JJY, Cubillan LD, Arroyo MH. Baseline Macular Pigment Optical Density among Filipinos with Age-related Macular Degeneration. Philipp J Ophthalmol 2014;39(2).
- Mupas J, Eusebio J Jr., Javate R, Pablo E Jr. Macular Pigment Optical Density in Healthy Eyes of Filipino Adults. Philipp J Ophthalmol 2015;40:93-96.
- Corvi F, Souied EH, Falfoul Y, et al. Pilot evaluation of short-term changes in macular pigment and retinal sensitivity in different phenotypes of early age-related macular degeneration after carotenoid supplementation. Br J Ophthalmol 2017;101(6):770-773.
- Taylor HR, West S, Muñoz B, et al. The long-term effects of visible light on the eye. Arch Ophthalmol 1992;110(1):99- 104.
- Taylor HR, Muñoz B, West S, et al. Visible light and risk of age-related macular degeneration. Trans Am Ophthalmol Soc 1990;88:163-73; discussion 173-8.
- Ho L, Boekhoorn SS, Liana, et al. Cataract surgery and the risk of aging macula disorder: the rotterdam study. Invest Ophthalmol Vis Sci 2008;49(11):4795-4800.
- Kara-Junior N, Espindola RF, Gomes BA, et al. Effects of blue light-filtering intraocular lenses on the macula, contrast sensitivity, and color vision after a long-term follow-up. J Cataract Refract Surg 2011;37(12):2115-2119.
- Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr 1995;62(6):1448S-1461S.
- Mukhtar S, Ambati BK. The value of nutritional supplements in treating Age-Related Macular Degeneration: a review of the literature. Int Ophthalmol 2019;39(12):2975-2983.
- Weigert G, Kaya S, Pemp B, et al. Effects of lutein supplementation on macular pigment optical density and visual acuity in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci 2011;52(11):8174-8178.
- Dimitrov PN, Robman LD, Varsamidis M, et al. Visual Function Tests as Potential Biomarkers in Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 2011;52(13):9457- 69.
- Fleckenstein M, Mitchell P, Freund KB, et al. The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology 2018;125(3):369-390.

