Risk Factors for Conjunctival Tube Erosion in Eyes Implanted with Ahmed Glaucoma Valve in a Private Eye Institution in the Philippines
Jovell Ian M. Peregrino, MD, DPBO, Edgar U. Leuenberger, MD, DPBO, Ma. Imelda Y. Veloso, MD, DPBO
Glaucoma drainage devices (GDD) are essential in the management of refractory glaucoma. Indications include glaucoma types with high rates of trabeculectomy failure (i.e. neovascular, uveitic, aphakic, and pseudophakic glaucoma) and in the presence of conjunctival scarring from previous intraocular surgeries including failed trabeculectomies. Results from the tube versus trabeculectomy (TVT) study have inspired better confidence in the use of glaucoma drainage devices. In the said study, a higher success rate was observed in the tube implant group compared to the trabeculectomy group. While similar reductions in intraocular pressure (IOP) were seen in both groups, the trabeculectomy group required more additional glaucoma surgeries for IOP control.
A review of literature yields numerous studies on the efficacy and safety of GDDs.1,7-9,10-11 However, despite the reports on successes of GDDs, there are serious drawbacks. Tube exposure through eroded conjunctiva is one of the more severe complications of GDD surgeries.7,12 The rate of conjunctival tube erosion (CTE) after GDD surgery ranges from 0.8 to 6.3%. CTE is identified as a major risk factor for endophthalmitis following GDD implantation. In a study by Al Torbak et al. consisting of 542 eyes implanted with GDD, endophthalmitis developed in 9 eyes (1.7%). In 6 out of 9 cases, CTE was present.
Determinants for the development of CTE include age, gender, ethnicity, orbital dimension, presence of health comorbidities, diagnosis, chronic use of topical medications, number of previous and concomitant ocular surgeries, and type of patch graft used.13-16 Nevertheless the strengths of association of the risk factors have not been established. A study by Muir and colleagues identified gender as the strongest risk factor while Trubnik et al. found concomitant ocular procedures with GDD are associated with CTEs. Such lack of consensus led us to investigate and identify the risk factors for CTE in eyes implanted with the Ahmed glaucoma valve relevant to our local setting. It is hoped that by doing so, the risk of developing CTE can be reduced. No similar studies were identified using the different search engines such as PubMed, Google Scholar, and the Philippine Journal of Ophthalmology archives. The results of this study can serve as a guide in performing GDD implantations specifically for the AGVs to minimize complications and increase the success of such procedures.
METHODS
This was a retrospective study of patients who underwent AGV implantation in a private eye institution from January 2004 to December 2013. Only charts with complete information and a minimum of 24-month postoperative follow-up were included in the study.
Information during the pre-, peri-, and postoperative periods were collected from the medical records. Preoperative data included age, gender, presence of comorbidities (diabetes mellitus [DM], hypertension), number of previous intraocular surgery, preoperative IOP, and indication for AGV implantation surgery. Previous intraocular surgery included trabeculectomy, penetrating keratoplasty(PKP), cataract surgery, repair of corneal laceration, and vitrectomy. Perioperative data included date of surgery and type of graft material used. The date of surgery was classified as having been performed in the dry/summer months (March to June) or wet/cold months (July to February). Postoperative data included immediate duration of follow-up, postoperative IOP, presence and duration of hypertensive phase, number of postoperative antiglaucoma medications, application and duration of digital massage, presence of blepharitis, use of bandage contact lens (BCL), and date of CTE occurrence. In addition, the number of major intraocular surgeries before the occurrence of CTE and the number of months from AGV implantation to CTE occurrence were also noted.
The primary outcome variable in this study was occurrence of CTE after AGV surgery. CTE was defined as exposure of any part of the tube occurring after one month of the surgery. This is differentiated from wound dehiscence which occurs within the first postoperative month period.13 Figure 1 shows an eye with intact conjunctiva over the tube implant, while figure 2 illustrates CTE.
Surgical Procedure
All surgeries involved implantation of an Ahmed® glaucoma valve (New World Medical, Rancho Cucamonga, CA, USA). The AGV model FP7 was used in adults, while the FP8 model was implanted in pediatric eyes. Surgeries were performed by one of 2 glaucoma surgeons with very similar surgical techniques. The operative technique is described below.
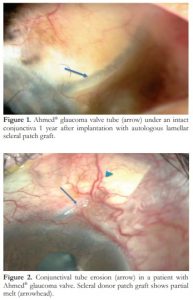
Figure 1. Ahmed® glaucoma valve tube (arrow) under an intact conjunctiva 1 year after implantation with autologous lamellar scleral patch graft.
Figure 2. Conjunctival tube erosion (arrow) in a patient with Ahmed® glaucoma valve. Scleral donor patch graft shows partial melt (arrowhead).
After intravenous sedation and general anesthesia were given for adults and children respectively, proper aseptic technique, and instillation of proparacaine eye drops, a limbal corneal traction suture using a Vicryl or nylon 7-0 or 8-0 was applied over the superotemporal limbus. A 1-mm conjunctival opening was made to bare sclera over the limbus to allow injection of lidocaine to the sub-Tenon’s space with a blunt cannula. This was followed by making a 3-clock hour conjunctival and Tenon’s incision over the superotemporal limbus to create a superotemporal fornix-based conjunctival flap located between the superior and lateral recti muscles. Using tenotomy scissors, the dissection was carried towards the equator to create a pocket for the AGV plate. The pocket was then irrigated with lidocaine to provide additional anesthesia. Hemostasis was achieved with bipolar cautery. The AGV was gently primed with balanced salt solution using a 27-gauge cannula. The plate was then inserted into the posterior pocket with its anterior border positioned 8-mm away from the limbus. Vicryl 8-0 and nylon 9-0 sutures were passed through the 2 plate eyelets to anchor the plate to the underlying sclera. A 23-gauge needle was used to create the scleral tube-track starting from 2-mm posterior to the limbus entering the anterior chamber. Viscoelastic was then injected through the tube-track to deepen the anterior chamber. The tube was then trimmed to adequate length, inserted bevel-up into the anterior chamber, and anchored to the sclera with interrupted 9-0 nylon sutures. The external tube was covered with either a 7×7 mm limbal-based 50-75% thick lamellar-autologous scleral flap or processed pericardium (Tutoplast®, Katena, Stewart Ct. Danville, NJ) cut to size. An autologous scleral patch was used when financial resources were limited. The tube was covered with the chosen graft anchored to the sclera by 2 to 4 interrupted nylon 10-0 sutures. The conjunctiva and Tenon’s were draped over the tube patch and reattached to the limbus with interrupted 10-0 nylon sutures. Vancomycin was then used to wash the operative field. Finally, triamcinolone acetonide was injected subconjunctivally over the plate.
Atropine, steroid, and antibiotic eye drops were used as postoperative medications.
Statistical Analysis
Descriptive statistics were used. Frequencies and proportions were generated for categorical variables. Means, standard deviations, and medians were computed for continuous variables. Fisher’s exact tests for proportions and independent t-tests were used to compare the groups with and without CTE. Univariable and multivariable logistic regressions were used to uncover associations between potential explanatory factors and outcomes. Odds ratio (OR) and confidence intervals (CI) were calculated. Survival analysis was used to compare the time to exposure for specific exploratory variables. Data were analyzed using a trial version of Stata IC version 13. P -values less than 0.10 were considered marginally significant, while P -values less than 0.05 were considered statistically significant.
RESULTS
Ninety-eight (98) eyes of 96 patients underwent AGV implantation from January 2004 to December 2013. Forty-six (46) eyes of 45 patients were included in the study. The mean duration of follow-up after GDD surgery was 50.2 months (range: 24 – 140 months, median: 44.5 months). Surgeries were mostly performed during wet/cold season (84.8%). Mean age was 55.9 ± 19.0 years old. About 43.5% were female. Diabetic patients comprised 34.8% of the sample while hypertensive patients made up 56.5%. The top indication for surgery was failed bleb (39.1%) followed by neovascular glaucoma (NVG) (21.7%). Most patients (76.1%) eyes received autologous scleral flap (Table 1).
Table 1. Patient Demographics and Baseline Characteristics of All Eyes Included in the Study

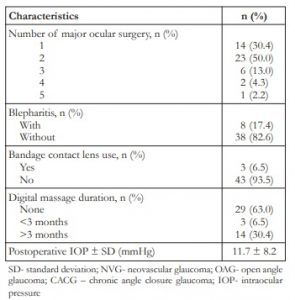
SD- standard deviation; NVG- neovascular glaucoma; OAG- open angle glaucoma; CACG – chronic angle closure glaucoma; IOP- intraocular pressure
Eight (8) eyes developed CTE (17.4%). CTE rates were significantly higher in eyes who had chronic angle closure glaucoma (CACG) (P =0.027) and in eyes which were required application of BCL in the postoperative period (P =0.004). CTE rates were also marginally significantly higher in females (P =0.056) and diabetic patients (P=0.083) (Table 2).
Table 2. Comparison of AGV Implanted Eyes With and Without Conjunctival Tube Erosion (CTE)


SD- standard deviation; NVG- neovascular glaucoma; OAG- open angle glaucoma; CACG – chronic angle closure glaucoma; IOP- intraocular pressure
In the univariable logistic regression analysis, only gender (P =0.064), diabetes (P =0.083), number of postoperative IOP-lowering eye drops (P =0.086), and number of major intraocular surgeries (P =0.09) were noted to be marginally significant. Specifically, females were found to have 5.1 times greater chance of developing CTE as compared to males, while diabetic patients were found to have 4.1 times greater chance of developing CTE as compared to nondiabetic patients. On the other hand, every increase in the number of IOP-lowering eye drops was found to increase the chance of developing CTE by 1.9 times. Lastly, every additional major intraocular surgery done was found to increase the chance of developing CTE by 2 times (Table 3).
Table 3. Factors Associated with Conjunctival Tube Erosion after AGV Surgery based on Uni-variable Logistic Regression Analysis

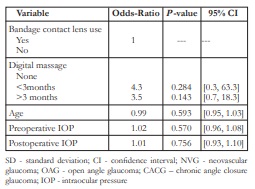
SD- standard deviation; NVG- neovascular glaucoma; OAG- open angle glaucoma; CACG – chronic angle closure glaucoma; IOP- intraocular pressure
For the multivariable analysis, only the variables with marginally significant P -values in the univariate analysis were entered (i.e. female gender, presence of DM, number of IOP-lowering eye drops, and number of major intraocular surgeries performed). Out of these four variables, only gender and presence of DM were found to be significantly associated with CTE (P <0.05). Specifically, females and diabetic patients were found to be 15.4 and 14.1 times more likely to experience CTE, respectively, holding all other factors constant (Table 4).
Table 4. Factors Associated with Conjunctival Tube Erosion after AGV Surgery based on Multivariable Logistic Regression Analysis
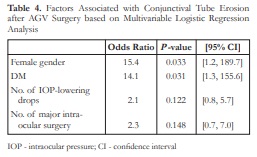
IOP – intraocular pressure; CI – confidence interval
The mean time from AGV implant surgery to exposure was 98.2 ± 12.9 months (median = 84 months). The mean time to exposure for females was 69.8 ± 7.0 months and for males was 128.3 ± 7.9 months. Survival analysis revealed that females experienced exposure of the AGV earlier in the course of follow-up than men (P =0.056). Survival of the GDD without exposure was not associated with presence of diabetes (P =0.115) (Table 5). Figure 3 illustrates estimated time (in months) from AGV surgery to tube exposure among men and women.
Table 5. Kaplan-Meier Estimates of Time to Exposure After AGV Surgery across Categories of Gender and DM Status
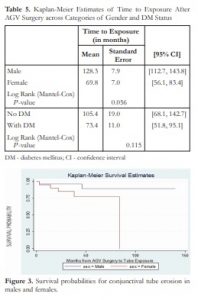
Figure 3. Survival probabilities for conjunctival tube erosion in males and females.
The 8 eyes with CTE were managed as follows. Two (2) eyes were managed conservatively with topical antibiotics and the tube erosion resolved. Six (6) eyes underwent additional surgical interventions. Three (3) eyes had processed pericardium graft with conjunctival autograft, 2 underwent scleral graft with autologous conjunctival graft, and 1 had repositioning of the tube to another quadrant. Of the patients who had processed pericardium re-graft, one developed recurrent CTE after 24 months and was managed with another processed pericardium re-graft with conjunctival autograft and responded well. The AGV of the patient who underwent tube repositioning was removed after 6 months due to development of preseptal cellulitis. The IOP was subsequently managed medically. One of the AGV implant patients without CTE developed endophthalmitis secondary to a central microbial keratitis and was treated accordingly.
DISCUSSION
Conjunctival tube exposure is one of the more common and serious complications of GDD. In this study, CTE was observed in 17.4% eyes that underwent AGV implantation surgery. Reported frequencies for CTE following AGV implantation ranges from 2-40%. Variations in CTE rates can be due to differences in patients’ characteristics and surgical technique.18-20
Several theories have been suggested to account for the development of CTE. These include chemical, anatomical, immunologic or mechanical factors such as micromovements of the tube during eye movements and blinking, and degenerative changes in the conjunctival tissue and Tenon’s capsule.12,20-23 In our study, one case of CTE occurred in the donor scleral flap group. This finding can be attributed to absolute alcohol, used as a preservative for donor sclera, and its toxic effects on ocular tissues24. Furthermore, it has been demonstrated that the absolute alcohol remains in the preserved donor sclera despite soaking in the saline solution.25
In our study, age was not associated with the development of CTE. This finding is similar to the observations of Trubnik et al. However, we noted that female gender turned out to be a significant risk factor based on multivariable logistic regression.16 Similarly, Muir et al. also showed an increased chance of developing CTE among females. These may be brought about by postmenopausal hormonal imbalance and a disparity in orbital dimensions between males and females.17,26 The smaller orbital dimensions in females may lead to additional microtrauma and friction between the eyelid and ocular tissues overlying the tube. Other studies, however, did not show significant associations between gender and CTE.15,16
In contrast to the findings of Koval et al. that noted NVG to be a risk factor, we found the presence of diabetes as a noteworthy determinant for the development of CTE.14 This divergence may be due to the small number of patients in our study whose indication for surgery was NVG (n = 10). Our result is defensible given that the vascular response of the conjunctiva is similar to that of the retina in patients with DM. A reduction in the vessel caliber with subsequent tortuosity can limit adequate perfusion in the conjunctiva which can result in ischemia and impaired wound healing causing poor conjunctival tissue strength, theoretically increasing the risk for CTE.27 Moreover, diabetes causes pathologic changes in the conjunctiva including squamous metaplasia, reduction in goblet cells, and increase in microaneurysms which can contribute to the friability of the conjunctiva.28 Diabetes has also been associated with a poor outcome in patients undergoing primary repair of CTE.31 Notwithstanding the preceding evidence, other studies did not find diabetes to be a risk for CTE.13,15-16,30 We recommend that the association between DM and CTE be validated in a prospective study given that the presence of DM among our cases was determined solely by chart review.
Based on univariable logistic regression analysis, we found a marginally significant association between the number of IOP-lowering drops with CTE. This result is similar to the findings of Geffen and colleagues except that they included the number of preoperative antiglaucoma drops while we took note of the number of antiglaucoma drops used postoperatively.30 IOP-lowering drops contain preservatives such as benzalkonium chloride which has toxic effects on the ocular surface. Ocular surface toxicity may manifest as squamous metaplasia, decrease in goblet cells, and destabilization of the lipid layer of the tear film causing rapid evaporation manifesting as dry eye disease.16,29
Although not routinely used after AGV surgeries, bandage contact lenses (BCL) were applied on 3 of our subjects who underwent concomitant penetrating keratoplasty (PKP). Unfortunately, all these 3 cases developed CTE, likely due to mechanical trauma and friction caused by the BCL on the underlying conjunctiva. Due to the very small number of cases, BCL use and blepharitis after AGV surgery were not found to be significant risk factors for CTE in our patients.
Previous ocular surgeries can lead to conjunctival scarring and thinning due to repeated inflammation. Univariable logistic regression in this study found that with every additional intraocular surgery performed, the chance of developing CTE increases two-fold. Similar studies found that a trabeculectomy prior to tube implantation was associated with a five-fold risk of CTE, while tube implantation with other concomitant eye surgeries showed a four-fold risk.14,15
The thickness and choice of graft material may influence the development of CTE. Lankarian et al. showed that a double thickness processed pericardium positioned over the silicone tube significantly reduced the incidence of CTE in mitomycin-augmented GDD surgery. Similar to the study of Smith, we found no statistically significant difference when comparing the incidence of CTE among our patients where an autologous patch graft or donor sclera or processed pericardium was used to cover the tube.20,21
We hypothesized that seasonal variations in environmental temperature and humidity may affect conjunctival health and the tendency to develop CTE. However, we found no significant association between month of surgery and CTE.
This study being a retrospective type has several limitations. The authors mainly relied on the availability of data in the charts which may not always be accurate. Additionally, only completely filled-out charts were included, thus limiting the sample size to only 46 cases. The differences in the surgical techniques of the 2 surgeons in this study were not accounted for in the analysis. Future studies may benefit from standardization of surgical techniques. Likewise, only postoperative IOP-lowering eye drops were noted in this study. The sample size may be in-creased by extending the study period and conducting an update of this study. A meta-analysis could also be done in future investigations. Diabetes and other comorbidities can be documented via evidence-based methods such as laboratory testing. IOP-lowering eye drops used both pre-operatively and postoperatively can be included and compared for differences. Future studies can be done to measure postoperative AC reaction quantitatively with the use of laser flare photometry.
Measures should be taken to reduce the risk of developing CTE. These include the use of tube patch materials that carry the lowest risk for erosions such as processed pericardium (Tutoplast®). In the study by Raviv et al. using pericardial patch grafts in all 44 cases, no CTE was observed on an average of
10.2 ± 4.0 months follow-up. However, patch graft melting without conjunctival erosion was seen in 4 cases.33 Creation of a scleral canal or trench that allows the tube to be fixed deeper into the scleral bed with the objective of lowering the overall tube elevation may be attempted in patients at risk for erosion.34,35 Measurements of the tube scleral distance by anterior segment optical coherence tomography showed that the tube was well within the sclera with no changes in the scleral contour or tenting of the overlying conjunctiva.35 Although technically challenging, creation of a longer needle-generated scleral tube tunnel by entering 4-6 mm posterior to the limbus may also offer a more viable alternative to autologous scleral flaps and patches. 36, 37, 38
In conclusion, females and diabetic patients were found to be more at risk of developing CTE. An increased number of postoperative IOP-lowering eye drops, and number of major intraocular surgeries also contribute to CTE. These risk factors should be taken into consideration when performing AGV implantation.
REFERENCES
1. Parihar JK, Vats DP, Maggon R, Mathur V et al. The efficiency of Ahmed glaucoma valve drainage devices in cases of adult refractory glaucoma in Indian eyes. Indian J Ophthalmol. 2009;57:345-350.
2. Dubey S, Prasanth B, Acharya MC, Narula R. Conjunctival erosion after glaucoma drainage device surgery: A feasible option. Indian J Ophthalmol. 2013;61:355-357.
3. Choudhari NS, Neog A, Sharma A, Iyer GK et al. Our experience of fibrin sealant- assisted implantation of Ahmed glaucoma valve. Indian J Ophthalmol. 2013;61:23-27.
4. Ainsworth G, Rotchford A, Dua HA, King AJ. A novel use of amniotic membrane in the management of tube exposure following tube shunt surgery. Br J Ophthal. 2006;90:417-419.
5. Rivera J, Leuenberger EU, Yap-Veloso MI. A retrospective review of autologous scleral flap versus donor scleral graft. Philipp J Ophthalmol. 2008;33:17-21.
6. Gedde SJ, Herdon LW, Brandt JD, Budenz DL et al. Postoperative complications in the tube versus trabeculectomy study during the five years follow up. Am J Ophthal. 2012;1535:804-814.
7. Minkler DS. Aqueous shunts in glaucoma AAO OTA. Ophthalmology. 2008;115:1089-1098.
8. Torbak AA, Shawn S, Jadaan I. Endophthalmitis associated with the Ahmed glaucoma valve implant. Br J Ophthalmol. 2005;89:454-458.
9. Lai JS, Poon AS, Chua JK, Tham CC et al. Efficacy and safety of Ahmed glaucoma valve implant in Chinese eyes with complicated glaucoma. Br J Ophthalmol. 2000;84:718-721.
10. Afrit MA, Trojet S, Mazlout H, Hamdouni M et al. Efficacy of the Ahmed glaucoma valve implant in eyes with refractory glaucoma. La Tunisale Medicale. 2007;85:941-944.
11. Wishart PK, Choudhary A, Wong D. Ahmed glaucoma valves in refractory glaucoma: a 7-year audit. Br J Ophthalmol. 2010;94:1174-9.
12. Oana S. Tube exposure repair. J Current Glau Prac. 2012;6:139-142.
13. Muir KW, Lim A, Stinnett S, Kuo A et al. Risk factors for exposure of glaucoma drainage devices: a retrospective observational study. BMJ Open. 2014;4:e004560.
14. Koval MS, Sayyad FF, Bell NP, Chuang AZ et al. Risk factors for tube shunt exposure: a matched case-control study. J Ophthalmol. 2013;196215.
15. Byun YS, Lee NY, Park CK. Risk Factors of implant exposure outside the conjunctiva after Ahmed glaucoma valve implantation. Japan J Ophthalmol. 2009;53:114-19.
16. Trubnik V, Zangalli C, Monster MR, Chia T et al. Evaluation of the risk factors for glaucoma drainage device-related erosion: A retrospective case control study. J Glaucoma. 2013;00:1-5.
17. Gedde SJ, Scott I, Tabandeh H, Lui KK et al. Late endophthalmitis associated with glaucoma drainage implants. Ophthalmology. 2001;108:1323-7.
18. Stewart WC, Kristoffersen CJ, Demos CM, Fsadni MG et al. Incidence of conjunctival exposure following drainage device implantation in patients with glaucoma. Eur J Ophthalmol. 2010;20:124-130.
19. Ayyala RS, Zurakowski D, Smith JA, Monshizadeh R et al. A clinical study of the Ahmed glaucoma valve implant in advanced glaucoma. Ophthalmology. 1998;105:1968-1976.
20. Lankaranian D, Reis R, Henderer JD. Comparison of single thickness and double thickness processed pericardium patch graft in glaucoma drainage device surgery. J Glaucoma. 2008;17:48-51.
21. Smith MF, Doyle JW, Ticrney JW Jr. A comparison of glaucoma drainage implant tube coverage. J Glaucoma. 2002;11:143-147.
22. Pakravan M, Yazdani S, Shahabi C, Yaseri M. Superior versus inferior Ahmed glaucoma valve implantation. Ophthalmology. 2001;108:1323-1327.
23. Heuer DK, Budenz D, Coleman A. Aqueous Shunt tube erosion. J Glaucoma. 2001;10:493-496.
24. Perkins TW, Kumar A, Kiland JA. Corneal decompensation following bleb revision with absolute alcohol: clinical pathological correlation. Arch Ophthalmol. 2006;124:738-741.
25. Enzenauer RW, Sieck EA, Vavra DE, Jacobs EP. Residual ethanol content of donor sclera after storage in 95% ethanol and saline rinse of various durations. Am J Ophthalmol. 1999;128:522-4.
26. Ferrario VF, Sforza C, Colombo A, Schmitz JH et al. Morphometry of the orbital region: a soft tissue study from adolescence to mid-adulthood. Plast Reconstr Surg. 2001;108:285-93.
27. Owen CG, Newsom RSB, Rudnicka AR, Ellis TJ et al. Vascular response of the bulbar conjunctiva to diabetes and elevated blood pressure. Ophthalmology. 2005;112:1801-1808.
28. Skarbez OD, Priestley Y, Hoepf M, Koevary S. Comprehensive review of the effects of diabetes on ocular health. Expert Rev Ophthalmol. 2010;5:557-577.
29. Huddleston SM, Feldman RM, Budenz DL, Bell NP et al. Aqueous shunt exposure: a retrospective review of repair outcome. J Glaucoma. 2013;22:433-438.
30. Geffen N, Buys YM, Smith M, Anraku A, Alasbali T et al. Conjunctival complication related to Ahmed glaucoma valve insertion. J Glaucoma. 2012;00:1-6.
31. Cvenkel B, Stunf S, Kirbis IS, Flezar MS. Symptoms and signs of ocular surface disease related to topical medication in patients with glaucoma. Clin Ophthalmol. 2015;9:625-631.
32. Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86:418-423.
33. Raviv T, Greenfield DS, Liebmann JM, Sidoti PA et al. Pericardial patch graft in glaucoma implant surgery. J Glaucoma. 1998;7:27-32.
34. Shaarawy T. New Advances in Glaucoma Surgery: Conjunctival- independent Glaucoma Surgery. Supplement to Glaucoma Today. 2011;6-7.
35. Bhartiya S, Shaarawy T. ASOCT Evaluation of deep scleral trench technique of Ahmed glaucoma valve implantation. World Glaucoma Congress. 2011, Paris.
36. Albis-Donado O, Gil-Carrasco F, Romero-Quijada R,Thomas R. Evaluation of Ahmed glaucoma valve implant-ation through a needle-generated scleral tunnel in Mexican children with glaucoma. Ind J Ophthalmol. 2010;58:365-73.
37. Gdih G, Jiang K. Graft-free Ahmed valve implantation through a 6 mm scleral tunnel. Can J Opthalmol. 2017;52:85-91.
38. Kugu S, Erdogan G, Sevim MS. Efficacy of long scleral tunnel technique in preventing Ahmed glaucoma valve through conjunctiva. Semin Ophthalmol. 2015;30:1-5.

