The Daily, Monthly, and Annual Cost of Glaucoma Therapy Using Ocular Hypotensive Eye Drops in the Philippines Based on a Quantitative Method
Corrina P. Azarcon, MD1, Nilo Vincent DG. Florcruz II, MD1,2,3
1Department of Ophthalmology and Visual Sciences, University of the Philippines – Philippine General Hospital, Taft Avenue, Manila
2University of the Philippines – College of Medicine, Taft Avenue, Ermita, Manila
3American Eye Center, 5/F Shangri-La Plaza, EDSA corner Shaw Boulevard, Ortigas Center, Mandaluyong City
Correspondence: Corrina P. Azarcon, MD
Sentro Oftalmologico Jose Rizal Building, Philippine General Hospital, Taft Avenue, Ermita, Manila
e-mail: corrinaa.md@gmail.com
Disclosure: No financial support was received for this study. The authors report no conflict of interests.
Glaucoma is listed among the top causes of irreversible blindness worldwide.1-3 Its estimated global prevalence was 64.3 million in 2013, with 60% of the diagnosed cases coming from Asia.1,2 The Third National Survey on Blindness in the Philippines reported glaucoma as the 3rd major cause of bilateral blindness and the 5th most common cause of low vision in the country.4
Studies have described the demographic and clinical profile of patients with glaucoma in the Philippines;5-6 however, socioeconomic factors and the financial burden of the disease have yet to be tackled. In many developed countries, the cost of glaucoma therapy is shouldered by the government and insurance companies.7-8 In contrast, out-of-pocket expenditure remains to be the major means by which medications are procured in the Philippines.
Drug price has been identified as a barrier to adherence to therapy.8-13 A study conducted in Canada showed that below-average income and lack of insurance were associated with higher rates of nonpersistence and more gaps in glaucoma therapy.14 Dreer et al. identified lower income, poor health, extremes of age, and African-American descent as risk factors for poor adherence to topical therapy among patients with glaucoma.15 Determinants of compliance to glaucoma medical therapy have not yet been described locally, although it would be rational to believe that increased costs would lead to decreased adherence to treatment in a country where maintenance glaucoma medications are an out-of-pocket expense.
Topical eye drops are the first-line therapy in the management of glaucoma.15 In a retrospective study by Rayel and Aquino (unpublished data), patients who eventually underwent glaucoma drainage device implantation were on an average of 2.6 topical medications prior to surgery. A higher mean of 3.2 was reported when systemic anti-glaucoma medications were included. Nearly 9 out of 10 patients were using β-blockers while 8 out of 10 patients were using αagonists before shunt implantation. The results of this study reflect the burden of glaucoma therapy.
The Philippine National Drug Formulary lists 8 topical ocular hypotensive or anti-glaucoma eye drops approved for the management of glaucoma – namely, pilocarpine, timolol, betaxolol, brimonidine, latanoprost, travoprost, brinzolamide, and dorzolamide.16 Over the past few decades, the number of available brands of eye drops in the local market has increased. The Food and Drug Administration of the Philippines lists several brands and formulations of ocular hypotensive medications approved for local use17; however, only the ceiling prices of timolol, latanoprost, and tafluprost have been identified in the latest versions of the Philippine Drug Price Reference Index.17-19 Multiple studies conducted in North America, China, Saudi Arabia, and India showed that bottle and solution properties are factors that affect drug administration and cost of therapy.20-24 No similar research has been done in the Philippines. The purpose of this paper is to determine and compare the projected costs of using different brands of ocular hypotensive eye drops that are readily available within Metro Manila.
METHODS
This was a laboratory research study conducted at the Central Laboratory of the National Institute of Health at the University of the Philippines – Manila. Eye drop brands were selected via convenience sampling based on the availability in major pharmacies within Metro Manila, Philippines. Only eye drops in multi-dose containers approved for use by the Food and Drug Administration of the Philippines for glaucoma were included. Preservative-free eye drops placed in single-use containers were excluded. All samples were stored under conditions recommended by the drug manufacturers. The prices used for the calculations were obtained from Mercury Drug, which is the largest pharmacy chain in the Philippines. Prices were updated as of April 2019.
The experiment was conducted at room temperature. A densitometric method based on the experiments by Enzenauer et al. and Moore et al. was employed in the calculation of drop density, drop volume, and bottle volume.24,25 All brands were randomly tested in triplicate samples. A single research assistant, masked from the study objectives, eye drop brands, and the parameters to be calculated, was assigned to dispense the drops on the weighing dish. For each sample, 10 drops of the ophthalmic solution were dispensed on a weighing dish. The dropping angle was set at 45o based on a similar study conducted in India.21 In between drop insillations, the bottles were held at a vertical position for at least three seconds. This process was repeated 5x to get 5 measurements of the mass of 10 drops.
The number of drops per bottle was determined by transferring the entire bottle contents onto the eighing dish one drop at a time and counting from first to last drop. Its corresponding mass was referred to as the total usable mass. Lastly, five readings of the mass of 200-microliter aliquot (mass of 200-µl aliquot) were recorded and the median value was identified.
At the end of the laboratory experiment, the following raw data were obtained: (1) mass of 10 drops, (2) total usable mass, (3) number of drops per bottle, and (4) mass of 200-µL aliquot.
The median mass (in grams) of the 200-microaliquot was divided by 0.200 mL to obtain the density of each sample. The total usable mass was divided by the calculated density to obtain the total usable volume. The total usable volume corresponds to the volume of the eye drop solution that was successfully transferred to the weighing dish. This value was representative of the amount of solution that can be used by a patient. The ideal result was a value greater than the declared volume of the bottle indicated in the bottle packaging. This would imply
ample overfilling.
Study Outcome Measures
Primary outcome measures included total usable volume, number of drops per milliliter, projected number of days that the bottles would last for unilateral and bilateral use, drop cost, and monthly and annual costs of therapy.
The number of drops per milliliter was calculated to assess bottle efficiency across all declared volumes. This is determined by dividing the total number of drops by the total usable volume. The projected numbers of days that the bottles would last for unilateral and bilateral use were also computed based on the number of drops per bottle. Drop cost was calculated for each brand by dividing the retail price by the number of drops per bottle. The daily cost was computed by multiplying the frequency of administration with the drop cost. In the determination of monthly costs and annual costs, 2 different sets of calculations were prepared based on 2 assumptions on how patients would use their eye drops. In the first set, it was assumed that the eye drops will be used until the bottle is empty. This is presumed to be more reflective of real-life practice in the Philippines. In the second set, it was assumed that the patient would conform to the manufacturer recommendation, which was to discard the eye drop bottle after one month of use.
Secondary outcome measures included brief physical descriptions of the eye drop bottles and its contents.
Data Analysis
Data are presented in medians with maximum and minimum values.
RESULTS
Twenty-one (21) bottle brands were included in the study. These consisted of 2 brands of α-agonists, 4 brands of β-blockers, 2 brands of carbonic anhydrase inhibitors, 5 brands of prostaglandin analogues, and 8 brands of fixed-combination eye drops. Sixteen (16) out of the 21 brands were innovator brands. The non-innovator brands were Alcon Brimonidine (brimonidine tartrate), Celsus Timolol (timolol maleate), Normopres (timolol maleate), Astapro (latanoprost), and Astimol (latanoprost + timolol maleate). The drug timolol had the greatest number of easily accessible preparations.
Twelve (12) of the 21 brands were packaged in 5-mL containers. Seven (7) brands were in 2.5-mL bottles, all of which were prostaglandin-containing solutions. Only 2 brands were in 3-mL preparations. Median values of the calculated densities, total usable volumes, drops per milliliter, drop volumes, and days per bottle are shown in Table 1. The range of the calculated densities was 0.920 to 1.011 g/mL. The smallest calculated drop volume was for Travatan (27.39 µL) and the highest was for Trusopt (50.15 µL). In general, the drop sizes of the brands of carbonic anhydrase inhibitiors and the α-agonists were larger than the drop sizes of the brands of β-blockers, prostaglandin analogues, and fixed-combination eye drops. An exception was Cosopt, a fixed-combination preparation, with a large drop volume of 40.83 µL. The range of the drop count for the samples were 93 – 172, 91 – 99, and 75 – 95 for the 5-mL, 3-mL, and 2.5-mL bottles, respectively. The number of drops per milliliter ranged from 20 to 36. The lowest and highest values were obtained for Trusopt and Travatan, respectively. The calculated values for the number of drops per milliliter were lower for the α-agonists and carbonic anhydrase inhibitors in comparison to the other drug groups; this was seen in correlation with the large drop volumes calculated for these two groups.

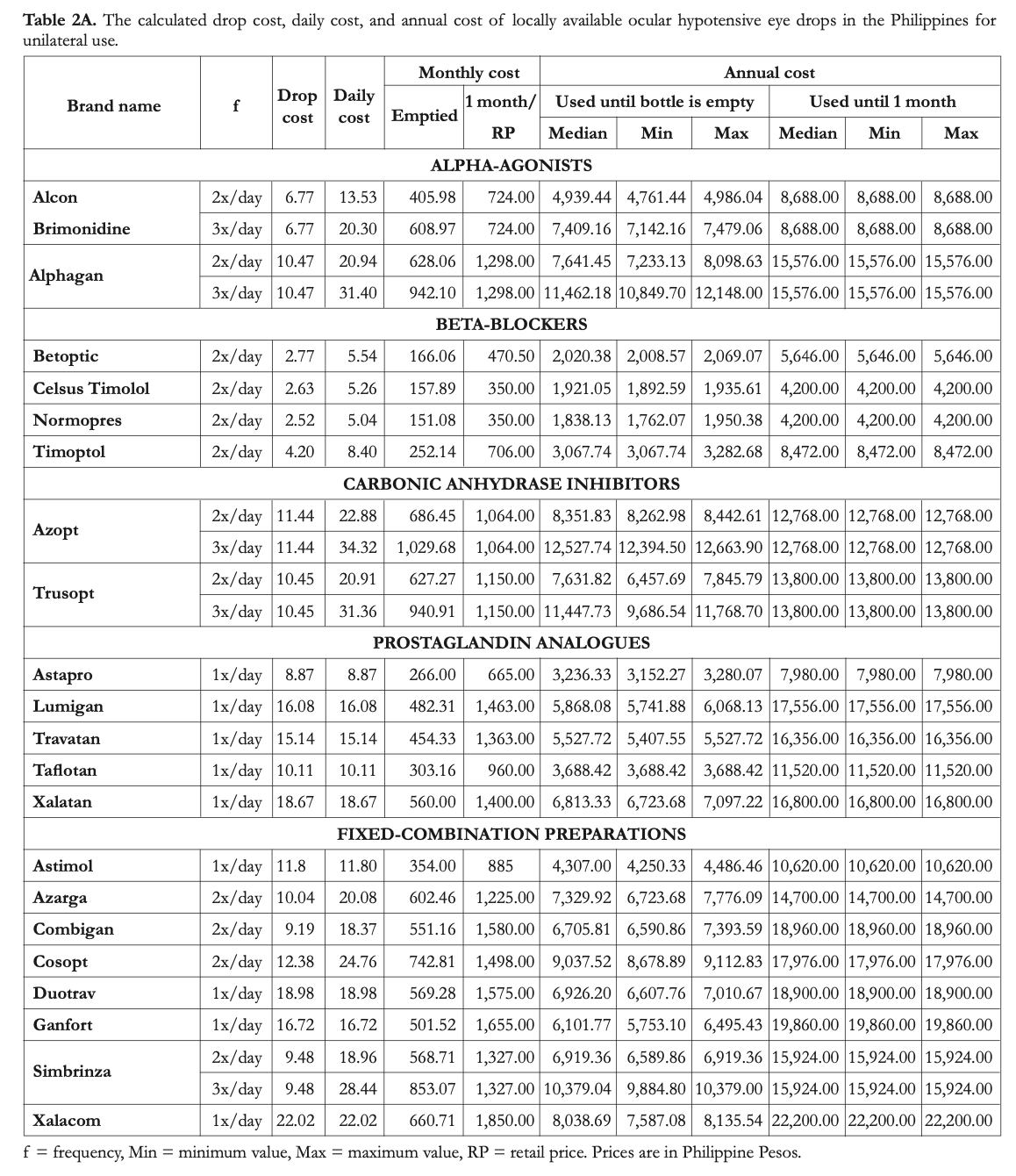
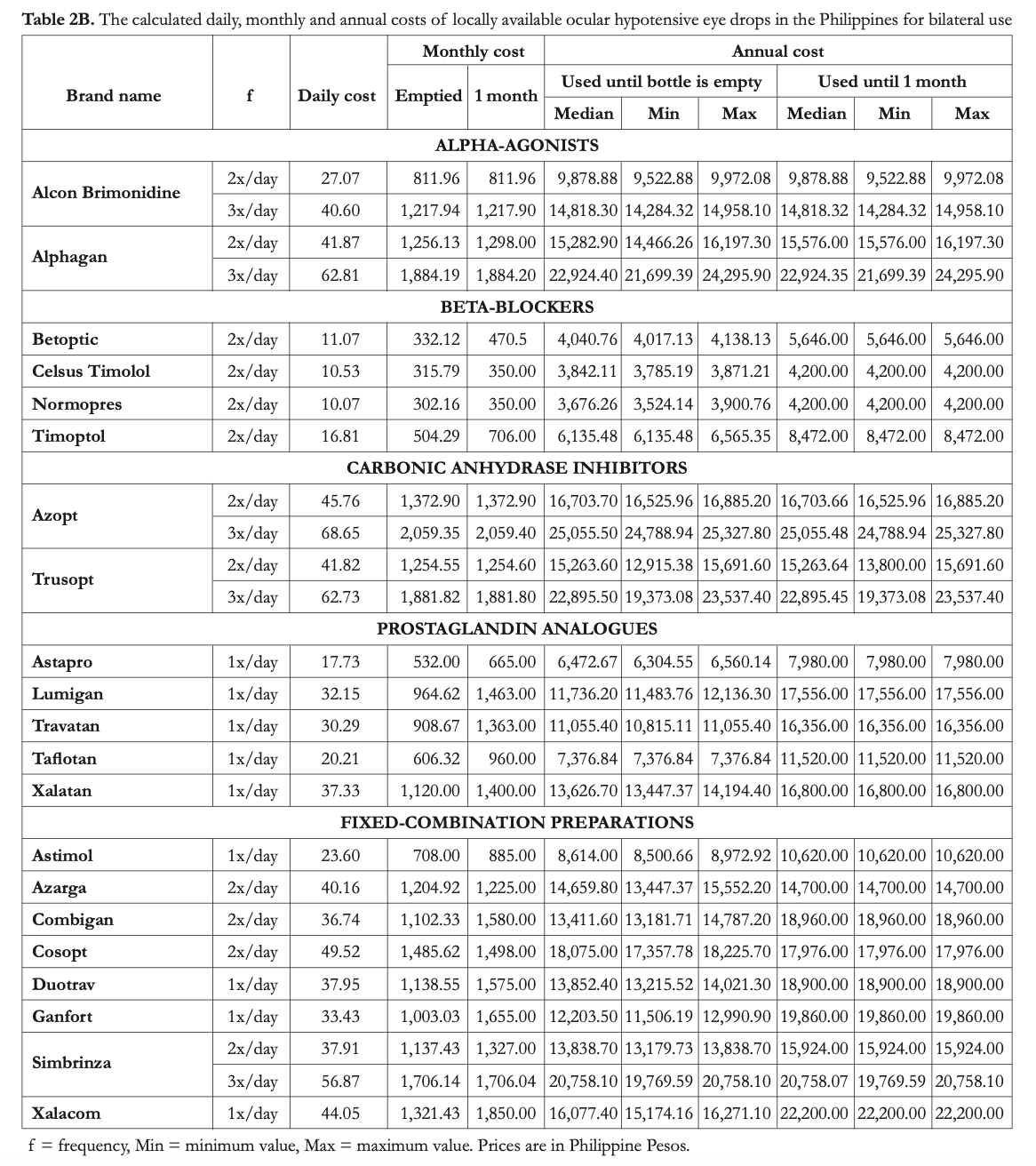

Our calculations show that none of the eye drop brands would be consumed in less than 30 days if used unilaterally. The range of the days per bottle if used only on one eye was 36 days (Alcon Brimonidine 3x/day) to 99 days (Ganfort 1x/day). The values computed for unilateral use were divided by 2 to obtain the days per bottle for bilateral use. Alcon Brimonidine (2-3x/day), Alphagan (3x/day), Azopt (2-3x/day), Trusopt (2-3x/day), and Simbrinza (3x/ day) would be consumed in less than 1 month if used for both eyes. The median number of days per bottle for bilateral use was highest for the prostaglandin analogues at 45 days per bottle (min: 38 days, max: 48 days) and lowest for the carbonic anhydrase inhibitors at 21 days per bottle (min: 16, max: 28). The median for α-agonists, β-blockers, and fixed-combination eye drops were 24 (min: 18, max: 27), 38 (min: 33, max: 42), and 38 (min: 23, max: 50) days per bottle, respectively.
The prices of the eye drops at the time of the study ranged from PhP350 to 1,850. Betablockers had the lowest mean retail price at PhP469. Prostaglandin analogues had the highest average retail price for the brands with a single active component at PhP1,170. The average retail price for the α-agonists and carbonic anhydrase inhibitors were PhP1,011 and 1,107, respectively. The average retail price of fixedcombination eye drops was Php1,505. The cheapest retail price for a fixed-combination preparation was PhP885 for Astimol, and the most expensive was PhP1,850 for Xalacom. Astimol and Xalacom are both preparations of latanoprost combined with timolol maleate.
The average cost of 1 drop for monotherapy ranged from PhP2.5 (Normopres) to 18.7 (Xalatan). For fixed-combination eye drops, the range was Php9.2 (Combigan) to 22 (Xalacom). The projected calculated costs are displayed in Tables 2A and 2B for unilateral and bilateral use, respectively.
Projected costs for monotherapy if the bottles were to be used up to the last drop were lowest for Normopres, Celsus Timolol, and Betoptic, which are all non-innovator beta-blockers. Meanwhile, the most expensive options are Azopt, Alphagan, and Trusopt, when used 3x daily. For fixed combinations, the cheapest option is Astimol and the most expensive option is Simbrinza used 3x/day. For each sample, the costs calculated for bilateral use were twice those obtained for unilateral use.
If the bottles were to be used only up to one month for one eye or for both eyes, Normopres, Celsus Timolol, and Betoptic remain to be thecheapest options. The most expensive options,
however, change depending if the drug is used on just one eye or on both eyes. For monotherapy on one eye, prostaglandin analogues, particularly Lumigan, Xalatan, and Travatan, which are all innovator brands, are the most expensive options. For monotherapy on both eyes, the two innovator carbonic anhydrase inhibitors, Azopt and Trusopt, and the innovator α-agonist, Alphagan, become the most expensive. For combination therapy, Astimol is still the cheapest option and Xalacom is the most expensive; both of these are combinations of latanoprost and timolol maleate.
Table 3 shows the cost of using the fixedcombination eye drops versus using different combinations of individual brands. Simbrinza was found to be the cheapest option for the combination of brimonidine and brinzolamide used twice daily. Astimol was the cheapest option for latanoprost combined with timolol. For timolol in combination with other prostaglandin analogues, the most affordable options were Duotrav (travoprost + timolol), Azarga (brinzolamide + timolol), Cosopt (Dorzolamide + timolol), and Ganfort (bimatoprost + timolol). For the combination of brimonidine and timolol, Combigan would be the cheapest if it were used up to the last drop. The generic combination of Alcon Brimonidine with either Normopres or Celsus Timolol was the cheapest option if the drugs were to be used only up to 1 month.
The composition of the containers and the physical descriptions of the solutions are shown in
Table 4. Fifteen (15) out 21 bottles are made of lowdensity polyethylene. Nearly all solutions were clear except for Azarga, Azopt, and Simbrinza, which were suspension formulations. Approximately half of the samples were watery in consistency.

DISCUSSION
This is a quantitative cost-analysis study conducted in a single research laboratory. Mass-density calculations were employed to determine the cost of different brands of ocular hypotensive agents available in Metro Manila, Philippines as of April 2019. Results show wide variations in the cost of therapy using different brands of ocular hypotensive eye drops. The factors that affect the projected costs included the following: retail prices, bottle design, frequency of use, drop volumes, and actual bottle volumes of the ophthalmic preparations.
The total usable volume of a bottle should allow use for approximately one month when used for both eyes, with ample allowance for losses due to minor spillage, evaporation, and failures in administration. Excessive volume allowances, however, may result to usage of the drug beyond the recommended period of one month. It would also signify that the bottle design and volume are not optimized, since a large proportion of the eye drop volume is only placed for wastage. Ten brands (Alphagan, Betoptic, Normopres, Timoptol, Trusopt, Travatan, Taflotan, Xalatan, Ganfort, Xalacom) out of the 21 brands have usable volumes that are greater than the volume specified on the bottle labels. Whereas, 11 out of 21 bottles (52.38%) were underfilled. This proportion is slightly better than that reported in India, where 62% of 245 bottles investigated were underfilled.21 Underfilling of bottles results to early exhaustion of eye drop solutions, which increases the annual cost of therapy.
A cross-sectional study reported that 1 for every 4 patients reported problems with early exhaustion of eye drop bottles. A third of the patients attributed this to bottle-related issues such as the expression of multiple or large drops. While the range of drop volumes was reported to be 25 -70 µL, the volume of the normal tear film is merely 7 µL. The fornix is only able to hold 30 µL of liquid without overflow.24,26 Thus, a considerable portion of the eye drop administered is actually wasted. The size of the drop depends on the following factors: bottle design and tip, solution properties, bottle position or angle, and the manner by which the bottle is squeezed. Moore reported that the economical bottle position actually varies across different bottle types.24 It has been concluded that a dropper tip with a small outer orifice diameter allows a consistent surface area and a smaller drop volume.24,26 The calculated drop volumes for each sample reported in this study are only median values. For a given eye drop solution, the actual drop volume changes depending on the properties of the bottle, amount of liquid and air inside, and the pressure exerted by an individual on the bottle when expressing the drop. It was observed that the sizes of the last few drops of solution were inconsistent due to the difficulty in expressing a complete drop when the volume of liquid was already depleted. This observation was in agreement with the finding of Gaynes, who said that the force required to express a drop from a bottle that is full is considerably less than that needed to express a drop from a nearly empty bottle.27 In this study, the largest drop volume computed was for Trusopt. Travatan had the smallest drop volume, which was approximately 45% of the value computed for Trusopt. Another notable observation was that different solutions placed in similar bottles yielded different drop volumes. Timolol, Trusopt, and Cosopt, when placed in Ocumeter Plus© bottles, dispensed different drop volumes. These variations were attributed to the differences in the chemical properties of the solution. This is a factor that should be taken into consideration by manufacturers when developing or selecting a container for their drug product. In addition, it was also observed that a significant amount of residual liquid was retained in the Ocumeter Plus© containers. This observation was similar to that reported by Gaynes et al.27
In literature, the optimal eye drop volume is set to only 20-µL due to the small capacity of the precorneal area and the risk for systemic absorption of the medications.21,29 All of the calculated drop volumes of the 21 drugs tested in this study had drop volumes greater than this value. If it were assumed that all the drugs have established therapeutic equivalence prior to approval of its sale in the market, it can be said that the brands with greater drop volumes also unnecessarily administer greater doses of both the active and inactive ingredients. Through reduction of the drop size to the optimal value, costs may be reduced both for the manufacturers and the end-users. The risk for adverse effects may also be minimized. This is another factor that manufacturing companies and regulating bodies should consider. At present, there are no guidelines that regulate bottle design or the amount of drops that are available per volume of medication.
Ikeda et al. stated that the annual cost of an eye drop is affected by the total number of drops and the manner of use.30 In this study, the number of days per bottle if all drops would be administered was computed for unilateral and bilateral use. Nearly all of the brands have labels or package inserts that advise patients to discard the bottle 4 weeks after opening. However, in the Philippines, patients tend to consume the contents of a bottle irrespective of the time it was opened. For this reason, 2 sets of calculations were made – one wherein it is assumed that a bottle will be used until it is empty, and another wherein it is assumed that a bottle will be discarded 1 month after it is opened. The first set depicts “real-life” use and the second shows “ideal use.” Based on calculations, none of the brands, when used and stored properly, are expected to be consumed within 4 weeks when used only for one eye. For bilateral use, Alcon Brimonidine, Alphagan (3x/day), Azopt, Simbrinza (3x/day), and Trusopt bottles will be consumed before the 4-week period. This signifies greater costs for patients who are maintained on these drugs.
Results of this study have shown that noninnovator brands of β-blockers are the cheapest options for topical monotherapy, regardless if it were used on one eye or on both eyes, and if the bottles were used up to the last drop or only up to one month. Significant variations in the overfill and the number of drops per milliliter were observed in this study, similar to the report of Fiscella.28 Although drug pricing is affected by several factors, optimization of the drug volume and bottle design is recommended to improve cost-efficiency. In general, if the drugs were to be used for only up to 1 month, the retail price would be the most significant determinant of the annual cost of therapy. The use of brands that lasts less than a month for bilateral use entails greater expenses for the patients maintained on these eye drops, as seen in the use of certain brands of α-agonists and carbonic anhydrase inhibitors. The volume allowance placed inside the bottles becomes an important factor if the bottles were used on both eyes up to the last drop. It is notable that the difference between the costs computed if the bottle were to be used until it is empty versus when it is discarded after one month was largest for the prostaglandin analogues and the fixed-combination preparations. Although certain brands of prostaglandin analogues, namely, Lumigan, Xalatan, and Travatan have the highest retail prices for single-agent preparations, they turn out to be less expensive than carbonic anhydrase inhibitors when used for both eyes and when used until emptied due to the larger volume allowance placed in these bottles. The large drop sizes of Azopt, Trusopt, and Alphagan make these drugs less cost-effective if the intention is to use the bottle up to the last drop. These drugs, when used thrice daily, also last less than a month for bilateral therapy, hence increasing cost of therapy. As Fiscella reported, bottles with smaller drop sizes may actually be cost-effective in the long run. Over the years, bottle designs have improved to produce smaller drop volumes and less wastage. Newer brands of medications were found to be more efficient, as measured in terms of the number of drops administered per bottle.28
The cost of using combination preparations was also compared to the cost of using two brands containing the individual drug agents. Calculations revealed that it is more cost-effective to use the combination preparations Simbrinza (brinzolamide + brimonidine tartate), Duotrav (travatan + timolol maleate), Azarga (brinzolamide + timolol), Cosopt (dorzolamide + timolol maleate), and Ganfort (bimatoprost + timolol) compared to using individual preparations of its components. Four of these combination preparations have timolol as an active drug. Although there are already several affordable non-innovator brands of timolol, its cheap cost is offset by the significantly more expensive price of the second anti-glaucoma drug. For the combination of brimonidine tartrate and timolol maleate, Combigan is cheapest option if it is used until the bottle is empty whereas Alcon Brimonidine used with either Celsus Timolol or Normopres is the most affordable option when it is used only up to 1 month. Xalatan and Xalacom are the first prostaglandin-containing products that became off-patent. Astimol, a newly available non-innovator combination preparation, is found to be the cheapest option for the combination of latanoprost and timolol. For the innovator options, it is more cost-effective to use Xalacom in comparison to Xalatan with Timoptol. It is important to remember, however, that the combination of a once-daily drug (i.e. prostaglandin analogues) with a twice-daily drug (i.e. β-blockers) would limit the recommended use of the combination drug to once-daily, hence the dosing of the β-blocker component would not be maximized. This should be taken into consideration when deciding on whether to prescribe combination or separate reparations. The use of fixed-combination eye drops may prove to be significantly more cost-effective in the future once more non-innovator brands are available in the market.
This study displays the large range of the cost of ocular hypotensive agents available in the Philippines. Gao et al. arrived at a similar conclusion in China, where timolol was also found to be the cheapest drug option.23 One limitation of this study is the single dropping angle used for analysis. Gaynes et al. conducted a densitometric assessment of topical antiglaucoma eye drops and found dosage variations that appear to be affected by the angle of administration.27 Gao stated that smaller drop volumes are produced when the bottle is squeezed in a vertical position. A slow and gentle squeeze results to a slow production of a drop, and prevents inadvertent expression of extra drops and larger drops.23 Moore, however, reported a contradicting finding. He reported that squeezing a bottle in a vertical position resulted to larger drops.24 In addition, a successful eye drop administration to one’s self requires a good hand-eye coordination, dexterity, and steadiness of the hand. Videotaped studies of eye drop instillation among glaucoma patients with impaired vision showed that for a single attempt of administration, a mean of 1.4 ± 1.0 drops are instilled using 1.2 ± 0.6 attempts.31 Calculations in this study assumed that all drops are instilled successfully. However, it can be said that patients who would have difficulties in self-instillation of eye drops may benefit from brands of the eyedrops with larger allowances for errors in instillation. For these patients, brands of β-blockers, prostaglandin analogues, and fixed-combination eye drops may be reasonable options, whereas certain brands of αagonists and carbonic anhydrase inhibitors would be less cost-effective. Patients who use the drugs on both eyes will also benefit significantly from greater overfilling.
Based on wages reported by the the Department of Labor and Employment in the Philippines, the annual income of a minimum-wage earner in the National Capital Region is 110,687.49 PhP.32 The range of the cost of therapy depending on the number of drugs used is displayed in Table 5. Analyzing this data alongside published local wages, it can be seen that patients can be spending as much as 3.3% to 66.9% of their annual income for their topical anti-glaucoma medications.

Cost of medications should be considered as an important factor in drug-selection along with the drug efficacy, side-effects, and frequency of administration.23 The calculations of this study reflect a best-case scenario for the use of the eye drops. Losses incurred due to streaming are accounted for in the methodology; however, the calculations do not reflect losses from misses during applications, evaporation, and spillage. Despite the limitations of a cost-minimization study, the findings may serve as a guide in understanding the cost-effectiveness of
the different brands of eye drops.23 It is necessary to educate patients on the proper use of eye drops in order to lessen cost of therapy.29
In conclusion, drop volume is a determinant of the number of drops that a bottle can administer; hence, drop volume affects the cost of therapy. Drop volumes for all 21 brands tested were greater than the ideal drop volume of 20 µL. Based on this study, only 10 of the brands had sufficient volume allowance. The most affordable brands belonged to the β-blocker drug class. Innovator brands of carbonic anhydrase inhibitors and α-agonists were found to be among the most-expensive options for monotherapy. Combination drug preparations have been found to be more cost-effective as compared to individual drug preparations when used together.
Data from this study allow physicians and patients to consider cost when choosing which drug preparation to purchase. This paper is only descriptive and does not make any assumptions with regards to the efficacy, safety, and chemical stability of any of the brands tested. It is recommended for drug developers to optimize the construction of bottles and placement of volumes in such a way that the drug will be consumed before the drug preparation declines in quality, with ample allowance for losses. It is recommended for future researchers to conduct
assays, bacteriologic and chemical studies to determine if it is safe and effective to use eye drops until the bottle is empty to minimize wastage and improve cost-effectiveness. Optimization of drug preparations is necessary to improve its cost-effectiveness, which in the long run would benefit both manufacturers and patients using the drug. Ultimately, there is a need for more generic preparations to enter the local market to decrease the prices of medications.
REFERENCES
1. Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-90.
2. Chan EW, Li X, Tham YC, et al. Glaucoma in Asia: regional prevalence variations and future projections. Br J Ophthalmol. 2016;100(1):78-85.
3. Quigley H, Broman A. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262-7.
4. Cubillan LDP, Olivar-Santos EO. Third national survey on blindness. Philipp J Ophthalmol. 2005; 30: 100-114.
5. FlorCruz II NV, Joaquin-Quino R, Silva PA, Khu P. Profile of glaucoma cases seen at a tertiary referral hospital. Philipp J Ophthalmol. 2005;30:161-165.
6. Martinez JM, Hosaka MB. Clinical profile and demographics of glaucoma patients managed in a Philippine tertiary hospital. Philipp J Ophthalmol. 2014;40:81-7.
7. Nayak B, Gupta S, Kumar G, et al. Socioeconomics of long-term glaucoma therapy in India. Indian J Ophthalmol. 2015;63(1):20-4.
8. Lazcano-Gomez G, Ramos-Cadena ML, Torres-Tamayo M, Hernandez de Oteyza A, Turati-Acosta M, Jimenez-Román J. Cost of glaucoma treatment in a developing country over a
5-year period. Medicine. 2016; 95(47):1-5.
9. Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surg. 1995 MayJun;26(3):233-6.
10. Sleath B, Robin AL, Covert D, et al. Patient-reported behavior and problems in using glaucoma medications. Ophthalmology. 2006;113(3):431-6.
11. Tsai JC. Medication adherence in glaucoma: approaches for optimizing patient compliance. Curr Opin Ophthalmol. 2006;17(2):190-5.
12. Tsai JC, McClure CA, Ramos SE, et al. Compliance barriers in glaucoma: a systematic classification. J Glaucoma. 2003;12(5):393-8.
13. Taylor SA, Galbraith SM, Mills RP. Causes of non-compliance with drug regimens in glaucoma patients: a qualitative study. J Ocul Pharmacol Ther. 2002;18(5):401-9.
14. Leung VC, Jin YP, Hatch W, et al. The relationship between sociodemographic factors and persistence with topical glaucoma medications. J Glaucoma. 2015;24(1):69-76.
15. Dreer LE, Girkin C, Mansberger SL. Determinants of medication adherence to topical glaucoma therapy. J Glaucoma. 2012;21(4):234-40.
16. Duque FT, Padilla AA, So RP, Quiambao DS, eds. Philippine National Drug Formulary Vol. 1. 8th ed. July 13, 2019: http://caro.doh.gov.ph/wp-content/uploads/2018/04/PNF-8thedition.pdf (accessed March 2, 2020).
17. Republic of the Philippines Food and Drug Administration. Registered Drugs. Feb 3 2018: http://www.fda.gov.ph/consumers-corner/registered-drugs-2 (accessed March 2, 2020).
18. Republic of the Philippines Department of Health. The Philippine Drug Price Reference Index, 6th ed. April 2019: https://dpri.doh.gov.ph/download/may/2018%20DPRI%20Booklet%20%20final%20 without%2010.pdf (accessed March 2, 2020).
19. Republic of the Philippines Department of Health. The Philippine Drug Price Reference Index. Feb 3, 2018: http://caro.doh.gov.ph/wp-content/uploads/2018/01/2017-
DPRI-Booklet.pdf (accessed March 2, 2020).
20. Al-Jumaian N, Malik R, Khandekar R, et al. Bottle characteristics of topical international glaucoma medications versus local brands in Saudi Arabia. Middle East Afr J Ophthalmol. 2016; 23(4): 296-301.
21. Banga H, Gupta A, Singh G. Volumetric and cost evaluation study of glaucoma medical therapy. Int J Appl Basic Med Res. 2015; 5(2): 96-99.
22. Mammo ZN, Flanagan JG, James DF, et al. Generic versus brand- name North American topical glaucoma drops. Can J Ophthalmol. 2012;47(1):55-61.
23. Gao Y, Wu L, Li A. Daily cost of glaucoma medications in China. J Glaucoma. 2007;16(7):594-7.
24. Moore DB, Beck J, Kryscio RJ. An objective assessment of the variability in number of drops per bottle of glaucoma medication. BMC Ophthalmol. 2017;17:78.
25. Enzenauer RW, Kao A, Williams T, et al. Relative Costs of Various Preserved Artificial Tear Solutions for the Treatment of Dry Eye Conditions. Eye Contact Lens. 2003;29(4):238- 40.
26. Van Santvliet L, Ludwig A. Determinants of eye drop size. Surv Ophthalmol. 2004;49(2):197-213.
27. Gaynes BI, Singa RM, Cao Y. Dosage variability of topical ocular hypotensive products: A densitometric assessment. J Glaucoma. 2009;18(2):149-52.
28. Fiscella RG, Green A, Patuszynski DH, et al. Medical therapy cost considerations for glaucoma. Am J Ophthalmol. 2003;136(1):18-25.
29. Sklubalova Z, Zatloukal Z. Systematic study of factors affecting eye drop size and dosing variability. Pharmazie. 2005;60(12):917-21.
30. Ikeda H, Tsukamoto H, Sawa A, et al. Comparison of annual cost between brand and generic ocular beta-adrenergic blockers. Yakugaku Zasshi. 2005;125(5):463-7.
31. Hennessy AL, Katz J, Covert D, et al. Videotaped evaluation of eyedrop instillation in glaucoma patients with visual impairment or moderate to severe visual field loss. Ophthalmology. 2010;117(12):2345-52.
32. Department of Labor and Employment. Current Real and Minimum Wages. Sep 18 2019: http://www.nwpc.dole. gov.ph/stats/current-real-minimum-wage-rates/ (accessed March 2, 2020).

