Safety and efficacy of intracameral triamcinolone in postcataract inflammation
Ma. Catherina G. Coronel, MD, George N. Co, MD
THE ADVENT of new phacoemulsification techniques, small-incision cataract surgery, and foldable intraocular lenses (IOLs) has not only shortened surgery time but reduced ocular trauma, as well. Patients, however, continue to experience significant postoperative inflammation. Corticosteroids, which reduce inflammatory exudation and inhibit the formation of fibroblasts and granulation tissue, have been the mainstay in controlling ocular inflammation. The use of topical steroids, which have been shown effective in controlling postoperative inflammation, has been the standard practice, although patient compliance remains an issue. Intravitreal, subtenon, and intracameral administration of steroids have also been employed, each with its own indications, advantages, and disadvantages. The use of triamcinolone acetonide has been gaining popularity in ophthalmic practice. This insoluble form of cortisone stays longer in intraocular fluids, like the vitreous and aqueous humor, making it ideal for controlling the inflammatory process if used intravitreally, intracamerally, or via subtenon’s injection. Some studies have reported traces of triamcinolone in the anterior chamber (AC) for as long as 6 months after intravitreal injection.
Triamcinolone has been used in the treatment of various ophthalmic conditions, such as cystoid macular edema, age-related macular degeneration (ARMD), and persistent uveitis. It has also been given subtenon’s to control postoperative inflammation. However, rare complications like scleral melt at the injection site and globe perforation have been reported.
Alternatively, triamcinolone has been injected intracamerally at the end of surger y to avoid these complications.
Other complications reported were increased intraocular pressure (IOP), development of cataract, endophthalmitis, and pseudoendophthalmitis. Problems of increased IOP postinjection have been recognized but are reportedly transient. Snow-globe effect causing pseudoendophthalmitis can be a problem, but is said to be dose-related and is distinguishable from AC inflammation. Intracameral administration of triamcinolone during surgery can help address patient-compliance problems as only antibiotic eye drops need to be given postoperatively. This study, therefore, evaluated the safety and efficacy of intracameral triamcinolone in controlling postoperative inflammation compared with topical steroid (1% prednisolone acetate). Complications and side effects were also evaluated.
METHODOLOGY
Eighteen eyes of 18 patients who underwent elective phacoemulsification with IOL implantation using 5.0 mm rigid polymethylmetacr ylate (PMMA) lens (Alcon) between June 2006 and August 2006 at the University of Santo Tomas Hospital and Quezon Institute were included in the study. Informed consent was obtained from all patients. Excluded were patients with glaucoma or preoperative IOP >22 mm Hg, those with history of ocular infection or inflammation or diabetes, those taking any oral or topical nonsteroidal antiinflammatory drugs (NSAIDs), and those who have had previous ocular surgery. Preoperative evaluation included visual-acuity testing, external-eye examination, slitlamp biomicroscopy, tonometry, and dilated-fundus examination. Patients were randomly assigned to 2 groups by drawing lots. The treatment group was injected with 0.4 mg triamcinolone in 0.1 ml solution and the control group with 0.1 ml of balanced salt solution (BSS) after removal of the ophthalmic viscosurgical device (OVD). The triamcinolone group was prescribed only topical antibiotic (0.3% ofloxacin) 1 drop every 2 hours for the first postoperative day, then 1 drop every 4 hours for 1 week, and 1 drop 4 times a day until 1 month. If inflammation remained significant 1 week postoperatively, topical steroid (1% prednisolone acetate) was given with the dosage depending on the degree of inflammation. Treatment failure of the triamcinolone group was defined as increasing amount of or nonimprovement of AC cells and flares and significant conjunctival hyperemia for at least 2 consecutive visits. The control group received 0.3% ofloxacin and 1% prednisolone acetate eye drops at 1 drop each every 2 hours for the first day, 1 drop every 4 hours for 1 week, and 1 drop 4 times a day for 1 month. All surgeries were done by the same surgeon using either the Millenium (Bausch and Lomb Storz) or the Legacy (Alcon Surgicals) phacoemulsification machines. Eyes were dilated with1% tropicamide prior to surgery and 2.5% phenylephrine every 15 minutes during surgery. Surgery was performed under topical anesthesia (0.5% proparacaine) and intracameral 1% lidocaine. All surgeries were performed in 2.75 mm clear cornea incision using phaco chop technique. Rigid 5.0 mm PMMA IOLs were used on all patients. After removal of the OVD, 0.4 mg in 0.1 ml solution of triamcinolone acetonide was injected intracamerally for the treatment group while 0.1 ml of BSS was injected intracamerally for the control group. Postoperative inflammation was graded according to the amount of AC cells, conjunctival hyperemia, clarity of cornea, and perception of symptoms of ocular inflammation, such as foreign-body sensation, photophobia, tearing, and eye pain. Patients were evaluated at baseline and in subsequent follow-up visits using slitlamp biomicroscopy without dilating the pupil. AC cells were graded as follows: 0 = no cells, 1 = 1 to 5 cells, 2 = 6 to 15 cells, 3 = 16 to 30 cells, 4 = >30 cells. Conjunctival hyperemia and corneal clarity were graded from 1 (mild) to 4 (very severe). Visual-acuity testing, applanation tonometry, slitlamp biomicroscopy, and ophthalmoscopy were done at each follow-up visit. Clinical assessment of ocular inflammation was conducted by a masked investigator and data were analyzed by a masked statistician. An intent-to-treat analysis was carried out. The Mann Whitney U Test was used to analyze variables with orderedresponse categories and continuous responses comparing the 2 treatment groups while the Friedman output was used to evaluate differences among repeated measures per treatment group. Significant difference was set at p value ≤0.05.
RESULTS
Of the 18 patients, 5 were males and 13 were females with a mean age of 64.6 ± 9.16 years (range, 32 to 73). Preoperative visual acuities ranged from 20/70 to hand movement while preoperative IOPs of both groups ranged from 10 to 18 mm Hg with a mean of 13.44 mm Hg. The demographic comparison between the 2 groups are shown in Table 1.
There was a significant difference between preoperative and postoperative VA in the control (p < 0.001) (Table 2). There was a significant difference in VA preoperatively and on the first day postoperatively (p = 0.01) in the triamcinolone group, but not in the succeeding weeks (p = 0.15).
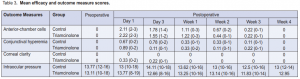
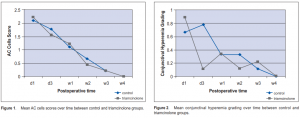
Significant difference in preoperative and postoperative AC cells in both the control (p <0.001) and triamcinolone (p = 0.001) groups was seen (Table 2), but no significant difference postoperatively between the 2 groups (0.98 vs 0.94 respectively) (Figure 1). There was a significant difference in conjunctival hyperemia preoperatively and postoperatively in the control (p = 0.02) and triamcinolone (p = 0.008) groups (Table 2). Between the 2 groups, a significant difference in the conjunctival hyperemia from day 1 to day 3 in the control and triamcinolone (p = 0.02) (Table 3) groups was seen. However, there was no significant difference between the groups on the succeeding postoperative days (Figure 2).
There was no significant difference in the corneal clarity preoperatively and postoperatively in both groups nor between the two treatment groups (Tables 2 and 3). There was a significant difference in the preoperative (13.77 mm Hg) and postoperative (13.21 mm Hg) IOP in the control (p = 0.03) but not in the triamcinolone (13.11 mm Hg and 12.93 mm Hg) (p = 0.15). Between the two groups, however, there was no significant difference in IOP preoperatively and throughout the postoperative days (Table 3).
DISCUSSION
The use of topical steroids as postoperative medication to control inflammation has been the standard practice in most ocular surgeries. Generally, this entailed instillation of drops several times a day plus other concomitant eye drops. Uncontrolled ocular inflammation can affect not only visual recovery but also the surgical outcome. The need for frequent instillation of eye drops can also affect compliance with postoperative treatment. Intracameral injection of triamcinolone at the close of surgery is aimed at reducing postoperative medications. Several techniques of delivering steroids intraocularly have been reported in the literature. Subtenon’s injection of triamcinolone for severe uveitis is an acceptable alternative and is also used in post-cataract surgery. Paganelli and coworkers found that subtenon’s injection of triamcinolone was not significantly different from topical 1% prednisolone acetate.
Complications of subtenon’s injection included globe perforation, subconjunctival hemorrhage, scleral melt, inadvertent injection into the retinal or choroidal circulation, central-retinal-artery occlusion, blepharoptosis, proptosis, orbital fat atrophy, strabismus, chemosis, and infection.
Intracameral triamcinolone to control post-cataractsurgery inflammation was studied by Gills and associates. The study showed that as the dosage of triamcinolone acetonide was increased, fewer eyes required topical steroids postoperatively (from 45% at the lowest dose to only 2% at the 1.8- to 2.1-mg level). None of the eyes that received 2.8 mg or more required additional steroid treatment. There was also a decrease in the rate of clinical CME as the dose of triamcinolone acetonide increased. Possible complications from intracameral injection included toxic anterior-segment syndrome (TASS), microstructural damage to corneal endothelium, and snow-globe effect in the AC, sometimes leading to pseudoendophthalmitis. These side effects can be prevented by proper preparation of the triamcinolone solution injected. In this study, particles adherent to the IOL were seen in some of the eyes, which eventually lessened. One patient had pseudohypopyon on postoperative day 1 that resolved on the third postoperative day. Others reported an increase in IOP after steroid injection, which was not seen in this study. There were no IOPs >18 mm Hg in both the control and triamcinolone groups. Endophthalmitis was also not observed in this study.
Overall, there was no significant difference in the scoring of AC cells, conjunctival hyperemia, and corneal clarity between the control and triamcinolone groups. In some patients, the conjunctival hyperemia may be less in the first 3 days postoperatively in the triamcinolone compared with the control group; in others, persistent inflammation requiring the use of topical 1% prednisolone acetate occurred. One had increased AC reaction during the first postoperative week and the other had ciliary flush during the third postoperative week. Only 1 from the control group had similar increase in postoperative inflammation that needed an increase in the frequency of prednisolone acetate.
Intracameral injection of triamcinolone can gain access to the vitreous cavity by flowing through the zonules. This can be an advantage in complicated surgeries, in those with posterior-capsular rupture to prevent cystoid macular edema, and in diabetic patients to reduce the risk of neovascularization and macular edema.
In summary, intracameral triamcinolone is comparable with topical steroids (1% prednisolone acetate) in controlling postoperative inflammation after cataract surgery. Posttreatment increase in IOP was not observed in this study. It can be a safe alternative to topical steroids as postoperative medication, reducing noncompliance and other problems like macular edema.
1. Jonas JB. Concentration of intravitreally injected triamcinolone acetonide in aqueous humour. Br J Ophthalmol 2002; 86: 1066.
2. Moshfeghi AA, Flynn HW. Complications of IVTA injection. Rev Ophthalmol 2004; 11: 12.
3. Gills JP. Avoiding complications with intraocular medications. Cataract and Refractive Surgery Today April 2005; 87-89.
4. Burk SE, Da Mata AP, Osher RH, et al. Triamcinolone-assisted anterior vitrectomy. Cataract and Refractive Surgery Today April 2005; 61-63.
5. Paganelli F, Cardillo JA, Melo LAS, et al. A single intraoperative sub-tenon’s capsule triamcinolone acetonide injection for the treatment of post-cataract surgery inflammation. Ophthalmology 2004; 111: 2102-2108.
6. Gills JP, Gills P. Effect of intracameral triamcinolone to control inflammation following cataract surgery. J Cataract Refract Surg 2005; 31: 1670–1671.
7. Beware potential complications with intracameral medications. Noor Vision Correction Center Ophthalmology Review. Daily Notes in Ophthalmology. October 28, 2005.
8. Oh JY, Wee WR, Lee JH, et al. Short-term effect of intracameral triamcinolone acetonide on corneal endothelium using the rabbit model. Eye 2006; 10: 1036.

