Retinal Toxicity and the Use of Hydroxychloroquine and Chloroquine in the Current Management of COVID-19
An Updated Advisory for Ophthalmologists and Other Health Care Professionals (March 28, 2020)
Romulo N. Aguilar, MD, PhD1 , Paolo Antonio S. Silva, MD2 , Recivall P. Salongcay, MD3 , Cheryl A. Arcinue, MD4 , Franz Marie Cruz, MD1 , Rory King Go, MD5 , Vitreo-Retina Society of the Philippines
1Department of Ophthalmology and Visual Sciences, College of Medicine, University of the Philippines, Manila
2Philippine Eye Research Institute, National Institutes of Health, University of the Philippines, Manila
3Eye and Vision Institute, The Medical City, Pasig City
4Asian Eye Institute, Makati City
5Chinese General Hospital, Manila
Correspondence: Romulo N. Aguilar, MD, PhD
Department of Ophthalmology and Visual Sciences, College of Medicine, University of the Philippines, Manila
e-mail: rnaguilar@up.edu.ph
Disclosure: The authors report no conflict of interests.
Chloroquine (CQ) and hydroxychloroquine (HCQ) are drugs that have been recommended for compassionate use in patients with moderate to severe COVID-19 infection. The Vitreo-Retina Society of the Philippines (VRSP), a sub-specialty society of the Philippine Academy of Ophthalmology (PAO), presents this advisory for the guidance of ophthalmologists and other health care professionals on the potential for retinal toxicity with the off-label use of the said medications for COVID-19 patients.
The risk of developing serious and irreversible retinal toxicity resulting in vision loss from long-term intake of HCQ or CQ is well documented.1-7 Currently, HCQ / CQ retinal toxicity has no known treatment. But, given the currently available clinical data, the risk for developing serious retinal toxicity among COVID-19 patients given HCQ or CQ is not fully known.1-8 Faced with these uncertainties, caution is advised and the following recommendations are made:
VRSP Recommendations:
• Prophylactic use of HCQ or CQ is not advised.
• When indicated, HCQ is preferred over CQ because it carries a lower risk of retinal toxicity.1,2
• There is currently no data to support the need for retinal screening tests and ophthalmic evaluation prior to initiating HCQ or CQ treatment of COVID-19 patients.7,8 However, patients must
be fully informed of the potential risk of retinal problems prior to initiation of therapy, if possible.
• Visual symptoms developing among COVID-19 patients under HCQ or CQ treatment should not prevent the continuation of an HCQ or CQ regimen.2,7,8
• COVID-19 patients with visual symptoms that persists beyond the treatment period may be referred to an ophthalmologist for proper evaluation at the proper time.
Disclaimer: These recommendations were formulated from the best currently available evidence, which involved long-term use of HCQ or CQ in non-COVID patients. These recommendations will be updated as new evidence is published, particularly with regard to COVID-19 patients.
Basis for recommendations:
Retinal toxicity from chloroquine (CQ) or hydroxychloroquine (HCQ) is a serious and irreversible ophthalmologic condition with no known treatment.1,2 The classic presentation is bilateral bull’s eye retinopathy. However, this is now less frequently encountered as current retinal imaging and diagnostic techniques in the current screening guidelines allow for the early diagnosis. Among Asian patients, initial damage may be seen in a more peripheral extramacular distribution near the vascular arcades.2 The risk of retinopathy increases with: (1) higher daily dose, and/or (2) prolonged treatment duration.1,2,6,7 This risk increases in the presence of renal disease, tamoxifen use, and underlying macular or retinal disease.2,6
The dose that is currently accepted as having the lowest risk of retinal toxicity is <5.0 mg/kg/day for HCQ. For CQ, the dose has been extrapolated and estimated to be <2.3 mg/kg/day.1,2,6,7
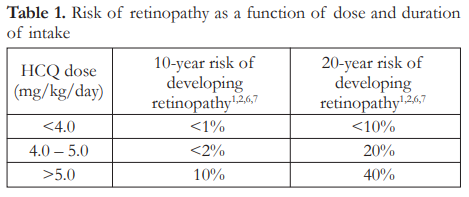
Most clinical studies from which data have been gathered involved subjects on extended intake of HCQ or CQ, namely patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), among others. These patients were maintained on these drugs over several months to years. Within the acceptable dose of <5 mg/kg/day, the 5-year risk of developing retinal toxicity is <1% and the 10-year risk is <2%.1,2,6,7 This increases to as high as 20% after 20 years (Table 1).1,2,6,7
In the current recommendations of the Philippine Society for Microbiology and Infectious Diseases (PSMID)9, the estimated daily dose the recommended <5 mg/kg/day. But, the recommendation is for a short duration of ten (10) days (Table 2).

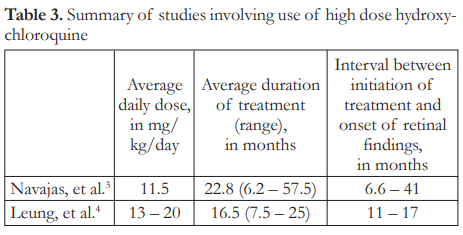
Higher doses of HCQ or CQ increase the risk of retinal toxicity.1-4,6,7 Daily dose ranging from 11.5 to 20 mg/kg/day, taken for 6 to 57 months, was associated with a 25% to 40% incidence of retinopathy in 1 to 2 years (Table 3).3,4 In one anecdotal report, retinal findings were seen as early as 2 months after intake of high dose HCQ.5 In this study, the patient was taking 400 mg of HCQ daily for 1 month and 200 mg daily for another month before signs of bull’s eye maculopathy appeared. In all these cited studies, the patients were all taking high dose HCQ for substantially more than 10 days.
Evidence linking retinal toxicity to HCQ / CQ use involves the extended use of the drug/s over a period of several months to years.1-4,6,7 Current use of HCQ or CQ in the management of COVID-19 patients is limited to ten (10) days or less. Presently, there is no data on the risk of retinal toxicity among patients on short course, high dose HCQ or CQ therapy such as that presently recommended for the treatment of COVID-19 patients.
Because most of the studies involved patients on long term HCQ or CQ therapy, and because HCQ or CQ retinopathy has an insidious onset, baseline ophthalmologic examination is recommended to be done within one (1) year of initiation of therapy, not necessarily prior to therapy.2,7 Annual screening starts after 5 years of HCQ or CQ therapy, unless other risk factors are present.2,7
For ophthalmologists: the following examinations have been recommended for screening purposes – (1) Fundus examination, (2) Visual field examination (for Asian patients: 24-2 or 30-2 visual fields; for non-Asian patients: 10-2 visual fields), (3) Spectral domain-optical coherence tomography or SD-OCT (widefield, if Asian). Optional examinations include: (1) Fundus autofluorescence (FAF), (2) Multifocal electroretinography (mfERG)
SUMMARY
There is strong evidence that HCQ or CQ can cause irreversible and non-treatable retinal toxicity that may lead to vision loss. Therefore, its prolonged use outside accepted indications is not advised. Currently available data involve the long-term use of HCQ or CQ on non-COVID patients. The risk for developing serious retinal toxicity among COVID-19 patients receiving HCQ or CQ, which are higher than standard doses, is presently not known but is likely low, given the relatively short course of treatment (10 days). Evidence also shows that retinal screening tests and ophthalmic evaluation is not necessary prior to initiating HCQ or CQ treatment. This recommendation becomes even more relevant when we consider the contagious nature of COVID-19. More definitive information on retinal toxicity and the use of HCQ or CQ in the management of COVID19 patients will be forthcoming. The short-term use of HCQ or CQ may be potentially lifesaving and the discontinuation of treatment solely on account of potential or suspected retinal toxicity may not be a reasonable option to take at this time.
REFERENCES
1. Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132(12):1453-60.
2. Marmor MF, Kellner U, Lai TY, et al. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology. 2016;123(6):1386-94.
3. Navajas EV, Krema H, Hammoudi DS, et al. Retinal toxicity of high-dose hydroxychloroquine in patients with chronic graft-versus-host disease. Can J Ophthalmol. 2015;50(6):442- 50.
4. Leung LS, Neal JW, Wakelee HA, et al. Rapid Onset of Retinal Toxicity From High-Dose Hydroxychloroquine Given for Cancer Therapy. Am J Ophthalmol. 2015;160(4):799-805 e1.
5. Pasaoglu I, Onmez FE. Macular toxicity after shortterm hydroxychloroquine therapy. Indian J Ophthalmol.
2019;67(2):289-92
6. Yusuf IH, Sharma S, Luqmani R, Downes SM. Hydroxychloroquine retinopathy. Eye (Lond). 2017;31(6):828–845. doi:10.1038/eye.2016.298
7. The Royal College of Ophthalmologists. Hydroxychloroquine and Chloroquine Retinopathy: Recommendations on Monitoring. January 2020: https://www.rcophth.ac.uk/wpcontent/uploads/2020/02/HCR-Recommendations-onMonitoring-Executive-Summary.pdf (accessed March 27, 2020).
8. Chodosh J, Holland GN, Yeh S. Important coronavirus updates for ophthalmologist. March 20, 2020: https://www.aao.org/headline/alert-important-coronavirus-context (accessed March 27, 2020).
9. Philippine Society for Microbiology and Infectious Diseases. INTERIM GUIDELINES ON THE CLINICAL
MANAGEMENT OF ADULT PATIENTS WITH SUSPECTED OR CONFIRMED COVID-19 INFECTION Version 2.0. March 26, 2020: https://www.psmid.org/cpgfor-covid-19-ver-2-updated-as-of-march-26-2020/ (accessed March 27, 2020).

