Rate of Progression of Visual Field Loss in Primary Open Angle Glaucoma Versus Primary Angle Closure Glaucoma Patients Managed in a Tertiary Hospital
Jesa Nadine V. Protasio, MD1, Nilo Vincent DG. FlorCruz II, MD2
1 Department of Ophthalmology
Davao Doctors Hospital
Quirino Avenue, Davao City, Davao del Sur
2 Department of Ophthalmology and Visual Sciences
Sentro Oftalmologico Jose Rizal, Philippine General Hospital
Taft Avenue, Ermita, Manila
Correspondence: Jesa Nadine V. Protasio, MD
Department of Ophthalmology
Davao Doctors Hospital
Quirino Avenue, Davao City, Davao del Sur
e-mail: jesanadine@yahoo.com
Disclosure: The authors declare no conflict of interest.
Glaucoma is the leading cause of irreversible blindness worldwide.1 It is a progressive neurodegenerative disease that results in damage to the optic nerve, with corresponding visual field loss. Assessment of visual field damage is of utmost importance and determining the rate of progression, measured in terms of visual field change per year (decibels (dB)/ year), influences management decisions.2
Glaucoma patients undergoing treatment are said to have a rate of progression of approximately 0.6 dB/year, a value between that of normal visual field decay (0.07 dB/year), and untreated glaucoma patients (1.1 dB/year).3 However, in a population of glaucoma patients and suspects under routine clinical care, while most progressed slowly at a rate <1 dB/year, 4.3% were fast progressors, identified as those with mean defect (MD) rates between 1-2 dB/year, and 1.5% were catastrophic progressors, with a MD rate >2dB/year.4 Studies that measure the rate of visual field progression of glaucoma patients undergoing treatment are important because they serve as benchmarks for clinical glaucoma care and allow clinicians to assess how successful they are in curbing the rate of disease progression and visual disability in their setting.
Several factors have been associated with an increased rate of visual field progression such as age, intraocular pressure (IOP), severity of baseline visual field MD, and glaucoma subtype but study results have been conflicting.4-9 Most of these studies only included open angle glaucoma types and need further validation. Identification of risk factors that influence the rate of visual field progression allows the clinician to pinpoint which patients are in need of more frequent monitoring or aggressive management.
The primary objective of this study was to determine and compare the rate of progression of visual field loss in terms of MD dB/year in primary open angle glaucoma (POAG) versus primary angle closure glaucoma (PACG) patients being treated at a tertiary hospital. In addition, the effects of baseline age, baseline MD, and IOP on the rate of progression of visual field loss were also assessed.
METHODOLOGY
This was a retrospective review of records conducted at the Department of Ophthalmology and Visual Sciences (DOVS) – Sentro Oftalmologico Jose Rizal (SOJR) of the Philippine General Hospital (PGH). The medical records of patients diagnosed with POAG and PACG who followed–up at the Glaucoma Clinic from August to October 2018 were reviewed according to the inclusion and exclusion criteria.
Eyes with POAG or PACG were included in the study. The diagnosis of POAG was made when there was evidence of optic nerve damage on clinical disc assessment/disc photo/ocular coherence tomography OCT), with angles open 360° on baseline gonioscopy, with at least one documented episode of IOP >21 mmHg, and no sign of any secondary mechanism responsible for the glaucoma. PACG was diagnosed when there was evidence of optic nerve damage on clinical disc assessment/disc photo/OCT, synechial or appositional angle closure ≥2 quadrants on baseline gonioscopy, peripheral anterior synechiae or at least one documented episode of IOP >21 mm Hg, and no sign of any secondary mechanism responsible for the glaucoma.
All patients were ≥40 years old at the time of diagnosis and baseline visual field examination, with visual acuity >20/200 throughout the duration of the study period (defined as the period encompassed between the first and last visual field tests analyzed). Corresponding medical records indicating IOP, addition of/change in medications, or if the patient underwent laser/surgery during the study period must also be available.
To be included, the study eye must have undergone ≥5 visual field examinations in ≥2 years using the G1 program (Dynamic Strategy) of the Octopus 311 Perimeter (Haag–Streit, USA), where each visual field examination included for analysis was classified as at least a Stage 1 based on the Enhanced Glaucoma Staging System (GSS 2) by Brusini11,12 and was deemed reliable. Reliability was defined as reliability factor ≤15%.
Eyes with a diagnosis other than POAG or PACG, or with an ocular disease that may influence visual field results (such as a vaso–occlusive disease, neurologic/neuro–ophthalmologic optic nerve pathology, diabetic retinopathy, retinitis pigmentosa, laser treatment on retina, pathologic myopia, tilted disc, retinal detachment, and macular degeneration) were excluded.
With regard to the visual fields, a test was excluded from analysis if it did not qualify as at least a Stage 1 based on the Enhanced Glaucoma Staging System (GSS 2) by Brusini10,11, if it was deemed unreliable, influenced by an artifact (i.e. clover leaf pattern, lens rim artifact, ptosis, or constricted pupil), associated with a 2-line decrease in visual acuity corresponding to an increase in cataract grade or worsening media opacity, or if the test was done <1 month after a laser or surgical procedure (including cataract surgery).
For each patient, both eyes were included to the study if both met the aforementioned criteria.
The following data was recorded from the medical records: diagnosis, number of reliable visual field tests, number of years followed (from the first to the last visual field test analyzed), baseline age (when the first visual field test included in the study was done), MD values of the first visual field test analyzed (baseline MD) and all subsequent visual field tests, and IOP at the time of each test (represented by the IOP on last follow-up prior to each visual field examination).
IOP-lowering interventions during the study period were also recorded, specifically medications, laser treatments, and surgeries. With regard to medications, the concept of the drug change score, adapted from the study of Heijl, was employed.5 The drug change score is the value that sums up all the changes in drug treatment during the course of the follow-up. At baseline, each patient received a drug change score of 0. For each addition/switch/removal of a medication, a point was added to the drug change score. (e.g. a patient who was on the same medication for the entire course of the study received a drug score of 0, while a patient who had five changes in medications received a drug score of 5). Laser treatments during the course of the study were noted; this referred to selective laser trabeculoplasty (SLT) for POAG patients and laser iridotomy (LI) or peripheral iridoplasty (PIR) for PACG patients. Cataract surgery and trabeculectomy, if performed during the study period, was documented as well.
For each study eye, the rate of visual field progression was calculated using linear regression analysis of the MD values over time, where rate of progression was the slope expressed in dB/year.
Descriptive analyses were performed on demographic data, follow-up duration, number of visual field tests, baseline MD, and IOP. T-test was done to compare the baseline data between the POAG and PACG populations as well as rates of progression in POAG and PACG. Pearson’s r correlation was done to evaluate factors hypothesized to affect the rate of visual field progression. Multivariate analysis was done to evaluate the effect of baseline age, baseline MD, mean IOP on follow-up, number of eligible automated visual fields (AVFs), and number of years followed, on the rate of progression of visual field loss for both the POAG and PACG populations. Since treatment has a direct effect on IOP, a second multivariate analysis was performed including the treatment variables. All statistical analysis was done using Stata Statistical Software (StataCorp LP. 2015. Stata Statistical Software: Release 14. College Station, Texas 77845 USA).
The primary outcome of the study was the rate of progression of visual field loss in MD dB/years in POAG and PACG patients managed in the Glaucoma Clinic of the PGH.
The secondary outcomes were the correlation between rate of progression of visual field loss and baseline age, baseline MD, and IOP (baseline, mean and fluctuation), as well as the multivariate analysis of rate of progression of visual field loss with baseline age, baseline MD, and IOP (baseline, mean and fluctuation) alone, and with treatment parameters (drug change score, laser, phacoemulsification, and trabeculectomy).
This study was approved by the University of the Philippines Manila Research Ethics Board (UPMREB) and was conducted in full conformance with the principles of the Declaration of Helsinki, Good Clinical Practice, and within laws and regulations of the University and the country.
RESULTS
A total of 92 eyes (78 patients) were included in the study. Thirty–three (33 or 36%) eyes had POAG, while 59 (64%) eyes were diagnosed with PACG (Table 1). Mean baseline age was 62.82 ± 7.78 years in the POAG group and 61.05 ± 7.84 years in the PACG group. The difference in mean age was not significantly different (p=0.3011) (Table 1).
A total of 781 AVFs were included in the study. The mean number of AVFs per study eye was 9.12 ± 3.28 in the POAG group and 8.14 ± 2.42 in the PACG group (p=0.10). The difference in the mean number of years followed (6.72 ± 2.85 and 5.37 ± 1.90 years in the POAG and PACG groups, respectively) was significant (p=0.0082) (Table 1).
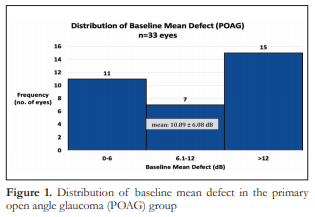
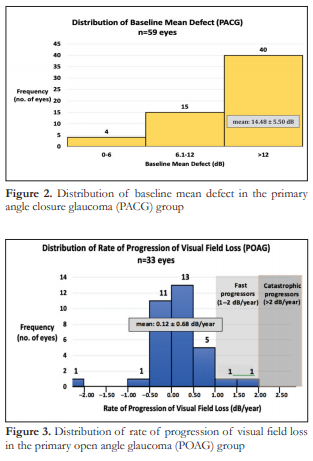
The entire spectrum of visual field loss from early, moderate to severe was represented in both groups. The mean baseline MD in the POAG group was 10.89 dB ± 6.08 which corresponds to moderate visual field loss (Figure 1), while the mean baseline MD in the PACG group was 14.48 dB ± 5.50 (Figure 2), which corresponds to severe visual field loss. The difference in mean baseline MD between the two groups was significant (p=0.0047) (Table 1).
Mean baseline IOP, mean follow–up IOP, and mean IOP fluctuation were 14.64 ± 4.66, 12.84 ± 2.52, and 17.27 ± 8.70 mmHg, respectively for the POAG group. For the PACG group, the values were 15.00 ± 7.40, 12.29 ± 2.60, and 18.98 ± 12.31 mmHg, respectively. None of the mean IOP measurements between the two groups was significantly different (Table 1).
With regard to treatment modalities, the mean drug change score was 5.61 ± 4.93 in the POAG group and 3.46 ± 4.12 in the PACG group. The difference between the two groups was significant (p=0.0280).
In the POAG group, 2 (6%) eyes underwent SLT, 9 (27%) eyes underwent phacoemulsification, and 15
(45%) underwent trabeculectomy. In the PACG group, 2 (3%) eyes underwent a laser procedure (1 LI, 1 PIR), 21 (36%) eyes underwent phacoemulsification, and 27 (46%) underwent trabeculectomy (Table 1).

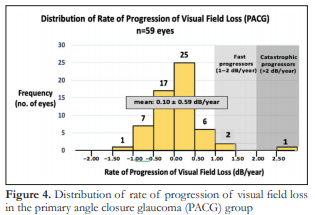
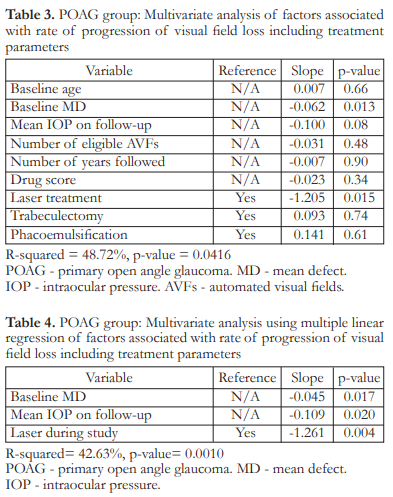
The rate of progression of visual field loss for the POAG group ranged from -2.4 dB/year to 1.97 dB/year with a mean 0.12 ± 0.68 dB/year (Figure 3). The rate of progression of visual field loss for the PACG group ranged from -1.19 dB/year to 2.7 dB/year with a mean of 0.10 ± 0.59 dB/year (Figure 4). The difference in mean rates of visual field progression between the two groups was not significant (p=0.8525).
Two (2) out of 33 (6.06%) POAG eyes were identified as fast progressors with rates of 1.2 and 1.97 dB/year. Two (2) out of 59 (3.39%) PACG eyes were identified as fast progressors with rates of 1.04 and 1.43 dB/year while 1 (1.69%) PACG eye was identified as a catastrophic progressor, with a rate of 2.7 dB/year. These fast and catastrophic progressors had 5–7 visual field examinations per eye, conducted within a span of 2.5 to 5.75 years.
In the POAG group, laser treatment was negatively correlated with rate of progression of visual field loss (r=-0.5072, p=0.0026). Baseline age, baseline MD, baseline IOP, mean IOP on follow–up, IOP fluctuation, drug change score, phacoemulsification, and trabeculectomy did not show any correlation with rate of visual field progression. In the PACG group, baseline MD (r=-0.2798, p=0.0318) and mean IOP on follow–up (r=0.368, p=0.0041) correlated with rate of progression of visual field loss. No correlation between rate of visual field progression and baseline age, baseline IOP, IOP fluctuation, drug change score, phacoemulsification, and trabeculectomy was found.
In the POAG group, multivariate analysis showed that a higher baseline MD (p=0.026) and mean IOP on follow–up (p=0.009) were associated with a significantly slower rate of progression (more negative slope). Baseline age, number of AVFs examined, and number of years followed were not significant (Table 2).

When the treatment parameters were added to the multivariate analysis, mean IOP on follow–up was no longer significant, but higher baseline MD (p=0.013) and laser treatment (p=0.015) were associated with a significantly slower rate of progression (Table 3). After one by one removal of non–significant variables from the analysis, we were left with the following significant variables: baseline MD (p=0.017), mean IOP on follow–up (p=0.020), and laser treatment (p=0.004). The model fit was statistically significant at p=0.0010, with an R2 of 0.4263 indicating that the independent variables explained 42.63% of the variation in rate of visual field progression (Table 4).
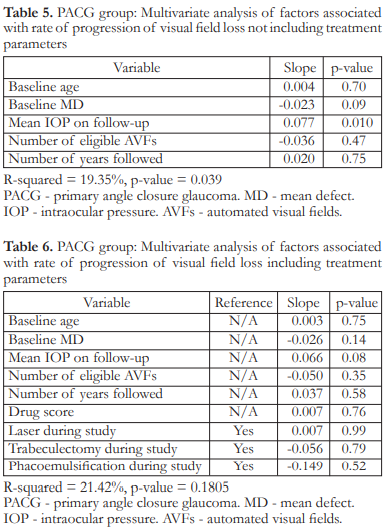
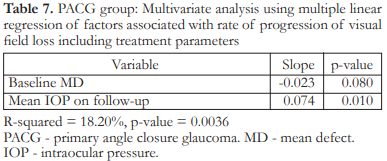
In the PACG group, multivariate analysis showed that a higher mean IOP on follow–up (p=0.010) was associated with a significantly higher rate of progression (more positive slope). Baseline age, baseline MD, number of AVFs examined, and follow-up duration were not significant factors (Table 5). When the treatment variables were added to the multivariate analysis, mean IOP on follow–up was no longer significant (Table 6). After removing non–significant variables one by one from the analysis until we arrived at a statistically significant model (p=0.0036), only mean IOP on follow–up was found to be significant (p=0.010). This model had an R2 of 0.1820 indicating that the independent variables explained 18.20% of the variation in rate of visual field progression (Table 7).
DISCUSSION
Rate of visual field progression was calculated using linear regression analysis of MD values over time and drawn as a slope in dB/year. The Octopus machine gives positive MD values where a higher or more positive value indicates more visual field loss. On linear regression, progression of visual field loss is indicated by a positive slope. This is in contrast with the Humphrey Visual Field Analyzer where a more negative MD value indicates more visual field loss. Hence, progression of visual field loss over time is indicated by a negative slope.
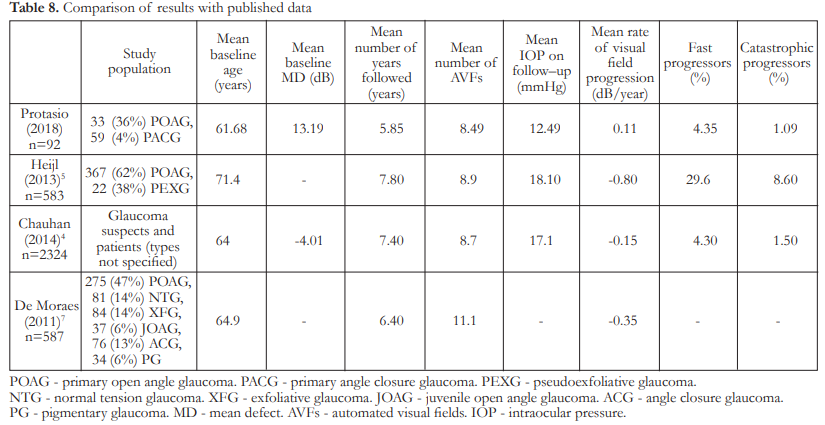
There was no significant difference in the rates of progression of visual field loss between POAG and PACG patients undergoing routine glaucoma care in our institution. Mean rate of progression in the POAG group was 0.12 ± 0.68 dB/year while that in the PACG group was 0.10 ± 0.59 dB/year (p=0.8525). This is comparable to the mean rate of visual field progression published in the study by Chauhan which is 0.15 dB/year, but much slower than the mean rates of progression reported by De Moraes and Heijl which were 0.35 dB/year and 0.80 dB/year respectively (Table 9).4,5,7 By defining fast and catastrophic progressors as those with rates of visual field change of 1–2 dB/year and >2dB/year respectively, our results are in agreement with other studies that showed that most glaucoma patients under routine clinical care progressed slowly (<1dB/year).4 A unique feature of the present study is that we compared the rates of visual field progression between POAG and PACG patients. This was made possible because of the high number of PACG patients seen in our institution. In comparison, the study by De Moraes had a study population of 587, but only 13% had angle closure glaucoma.7 No comparison was made between glaucoma subtypes. The study by Chauhan studied the rates of progression of glaucoma suspects and those with manifest glaucoma but did not segregate the latter according to types.4 The study by Heijl only involved POAG and pseudoexfoliative glaucoma (PEXG) patients.5 The aforementioned studies also reported data from AVF examinations done on the Humphrey Visual Field Analyzer. This is in contrast with our study where the visual field examinations were taken using the Octopus machine.
Despite treatment, a total of 4 (4.35%) eyes were identified as fast progressors while only 1 (1.09%) eye was identified as a catastrophic progressor. This is similar to the rates reported by Chauhan (4.3% fast and 1.5% catastrophic progressors) but much less than the proportions reported by Heijl (29.6% fast and 8.6% catastrophic progressors).4,5
The accuracy of the rate of visual field progression is related to several factors including length of follow–up and number of examinations. According to Chauhan, fewer AVF exams or a shorter follow–up period resulted in more outlier observations and that a greater number of examinations decreased the number of fast and catastrophic progressors.4 In agreement with this, the 5 eyes in our study that were identified as fast and catastrophic progressors only had 5–7 visual field examinations per eye conducted within a span of 2.5 to 5.75 years. The patients with rates of visual field progression >1 dB/year thus had a fewer number of analyzed visual field exams and shorter follow–up time compared to the rest of the study eyes. Perhaps more visual field examinations or a longer follow–up time will yield slower progression rates.
Analyzing the factors affecting the rate of visual field progression in POAG eyes showed that laser treatment, specifically SLT, during the study period revealed a moderately negative linear correlation (Pearson’s r=-0.5072, p=0.0026). This implies that SLT, an intervention that aims to lower IOP by improving aqueous outflow, is associated with a slower rate of visual field progression in POAG eyes. We take note however of the small sample size of 33 eyes where only 2 patients underwent SLT.
In the POAG group, multivariate analysis showed that a higher baseline MD and mean IOP on follow–
up were associated with a slower rate of progression. After plugging in the treatment variables, repeat multivariate analysis showed that mean IOP on follow– up was no longer significant while laser treatment and
baseline MD were significant factors. After removal of non–significant variables from the analysis to come up with a model that only includes significant predictors, baseline MD, mean IOP on follow–up, and laser treatment were found to be associated with a slower rate of visual field progression.
Higher baseline MD signifies more visual field loss. Its association with slower progression in POAG eyes can be due to truncation effects where a visual field with very advanced defects cannot progress as much as a field with smaller defects.5 Perhaps, a higher MD value at baseline also prompted more aggressive management resulting to a slower rate of progression. The same finding was also reported by Heijl.4,5
Higher mean IOP on follow–up was associated with a slower rate of visual field progression. This could be due to more aggressive IOP–lowering interventions. This finding might also be explained by the theory that POAG, compared to PACG, is hypothesized to be a less pressure–dependent disease. In a study by Gazzard, a weaker correlation between IOP and visual field loss was found among POAG patients compared to PACG patients.12 He postulated that other pressure-independent mechanisms may exist in eyes with POAG. Despite these findings, IOP still remains the sole proven modifiable risk factor for all types of glaucoma. Mean follow-up IOP was not found to be significant after multivariate analysis in the study by Chauhan. Heijl on the other hand found a higher mean IOP to be associated with faster rates of progression, which is in disagreement with the finding of our study.
Analyzing the factors affecting the rate of visual field progression in eyes with PACG showed that baseline MD was weakly negatively correlated (Pearson’s r=-0.2798, p=0.0318) while mean IOP on follow–up was weakly positively correlated (Pearson’s r=0.368, p=0.0041). These imply that a higher baseline MD was associated with a slower rate of progression in PACG eyes, again perhaps due to truncation effects or more aggressive management. A higher mean IOP on follow–up on the other hand was associated with a faster rate of progression (positive slope), the implications of which will be discussed below.
In the PACG group, multivariate analysis showed that only mean IOP on follow–up was associated with rate of progression but this effect was gone when treatment variables were added to the analysis. After removal of non–significant variables from the analysis to come up with a significant model, only mean IOP on follow–up was found to be significant.
The finding that higher mean IOP on follow–up was associated with a faster rate of progression in the PACG group might mean difficulty in controlling the IOP in this subset of patients, as compared to POAG. This also strengthens the claim of Gazzard that PACG may be considered to be a more purely pressure-dependent disease than POAG.12
Interestingly, our study did not find baseline age to be associated with rate of progression of visual field loss in both POAG or PACG groups. This is in contrast to the result of other studies that reported a correlation between older baseline age and faster progression.4,5 A younger mean age in our study population may partly explain the differences in our findings.
Since the study is retrospective in nature, we feel that this is an accurate reflection of routine clinical care because changes in management were not implemented according to changes in the rate of visual field progression (i.e. more aggressive IOP lowering schemes could have been done for eyes noted to have fast rates of progression, effectively slowing down the rate of progression from that point onwards, as would be assumed to happen in a prospective study). However, also because of its retrospective nature, there is a certain degree of variability inherent in the data.
CONCLUSION
Majority of the glaucoma patients being managed in the PGH have a slow rate of progression of visual field loss with POAG eyes that progress at a mean rate of 0.12 dB/year which is not significantly different from PACG eyes that progress at a mean rate of 0.10 dB/year. Despite undergoing treatment, 4.35% of study eyes were identified as fast progressors, while 1.09% were catastrophic progressors. Factors associated with rate of progression of visual field loss in a clinically treated population are baseline MD, mean IOP on follow–up, and laser done during the study period for POAG, while only mean IOP on follow–up was significantly associated for PACG.
ACKNOWLEDGMENTS
We would like to acknowledge the contributions of Mae Ann DL. Daduya from the School of Statistics, University of the Philippines, Diliman.
REFERENCES
1. Asia Pacific Glaucoma Society. Asia Pacific Glaucoma Guidelines, 3rd edition. The Netherlands: Kugler Publications, 2016.
2. Chauhan B, Garway-Heath D, Goñi F, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92:569-573.
3. White, A. Interpreting visual fields. https://www.slideshare. net/presmedaustralia/ccdh-optom-talk2014andrew-white32512696. Date published: March 19, 2014. Date accessed: August 13, 2019.
4. Chauhan B, Malik R, Shuba L, et al. Rates of glaucomatous visual field change in a large clinical population. Invest Ophthalmol Vis Sci. 2014;55:4135-4143.
5. Heijl A, Buchholz P, Norrgren G, Bengtssom B. Rates of visual field progression in clinical glaucoma care. Acta Ophthalmol. 2013;91:406-412.
6. Chauhan B, Mikelberg F, Artes P, et al. Canadian Glaucoma Study 3. Impact of risk factors and intraocular pressure reduction on the rates of visual field change. Arch Opthalmol. 2010;128(10):1249-1253.
7. De Moraes C, Juthani V, Liebmann J, et al. Risk factors for visual field progression in treated glaucoma. Arch Opthalmol. 2011;129(5):562-568.
8. Forchheimer I, De Moraes C, Teng C, et al. Baseline mean deviation and rates of visual field change in treated glaucoma patients. Eye 2011;25:626-632.
9. De Moraes C, Liebmann J, Liebmann C, et al. Visual field progression outcomes in glaucoma subtypes. Acta
Ophthalmol 2013;91:288-293.
10. Brusini P, Filacorda S. Enhanced glaucoma staging system (GSS 2) for classifying functional damage in glaucoma. J Glaucoma 2006;15:40-46.
11. Brusini P, Johnson C. Staging functional damage in glaucoma: review of different classification methods. Surv Ophthalmol 2007;52(2):156-179.
12. Gazzard G, Foster P, Devereux J, et al. Intraocular pressure and visual field loss in primary angle closure and primary open angle glaucomas. Br J Ophthalmol. 2003;87:720-725.
13. Smith S, Katz J, Quigley H. Analysis of progressive change in automated visual fields in glaucoma. Invest Ophthalmol Vis Sci. 1996;37(7):1419-1428.
14. European Glaucoma Society. Terminology and guidelines for glaucoma, 4th edition. Italy: PubliComm, 2014.

