PSCRS Guide to Ophthalmic Practice in the Times of COVID-19
(May 22, 2020)
Carlos G. Naval, MD, MBA1, Benjamin G. Cabrera, MD2
for the Philippine Society of Cataract and Refractive Surgery (PSCRS)
1 Galileo SurgiCenter, Mandaluyong City
2 American Eye Center, Mandaluyong City
Correspondence: Carlos G. Naval, MD, MBA
Galileo SurgiCenter
271 EDSA, Mandaluyong City
e-mail: cgnaval@gmail.com
Let’s begin by accepting reality. The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has abruptly disrupted our lives and, for some, it was fatal. Let’s be glad that we have survived thus far because the recursive path of the virus isn’t over yet. It’s not going to go away soon and it will never disappear. We have to learn to coexist with it. The coronavirus disease 2019 (COVID-19) crisis is playing out just like the Spanish Flu pandemic of 1918-1920. Eventually, the Spanish Flu fizzled out after killing about 50 million people worldwide, not because of a vaccine but because the majority of the rest of the population was immune to the virus and transmission stopped. If history were to repeat itself, the deadly strain of the SARS-CoV-2 will have killed its susceptible hosts and died along with them, and a less virulent strain will be endemic causing only mild symptoms except in rare cases and sprouting occasional outbreaks like influenza. In the meantime, we have to learn to cope with the times and adapt measures to prevent transmission to and from our patients and co-workers.
This guide is a compilation of recommendations culled from the knowledge of experts and currently available evidence as they relate to our practice presented as practical, commonsensical steps that can be applied in your clinics and your surgeries. We cannot claim to be comprehensive; although we tried to be thorough since many things about this virus are still in the process of being discovered. In fact, many of the recommendations out there are based on flimsy evidence and hypothetical situations contrived to simulate viral spread with aerosol sprays without really establishing validity of the construct. From time to time, please read the directives from the World Health Organization (WHO), the Department of Health (DOH), other government agencies, and health authorities who continue to study COVID-19.
Health Situation
The SARS-CoV-2 is highly transmissible and detected in tears and ocular surface but at very low rates.1 Conjunctival transmission is unknown and the eye as a route of infection by the virus is purely hypothetical.2, 3 Although there are numerous case reports of conjunctivitis and ocular surface disease as part of the presenting symptoms of COVID-19, there are no other ocular morbidities that have been clearly associated with it4; while retinal changes are theorized based on the expression of ACE2 receptors in the retina. Red eye, photophobia, epiphora and eye pain reported by non-hospitalized COVID-19-positive patients are more likely present in COVID-19-negative cohort of patients who also underwent testing.5 A laboratory study where viral replication of SARS-CoV-2 was demonstrated in cultures of ex-vivo conjunctiva proposes that the conjunctiva is a route of entry of the virus.6 In a published meta-analysis, the incidence of conjunctivitis was found to be 3% and 0.7% in severe and non-severe COVID-19 cases, respectively.7 In a large retrospective study of COVID-19 treated patients in Hubei Province, China, one third of patients manifested with conjunctivitis. Although, only 16.7% of confirmed COVID-19 patients by nasopharyngeal reverse-transcription polymerase chain reaction (RT- PCR) also had positive results from the conjunctival RT-PCR testing.8 A prospective study in Singapore on 17 RT-PCR positive COVID patients failed to show any evidence of the virus from systematic tear sampling even in the patient who exhibited conjunctival congestion and chemosis.9 Despite the scarcity of the evidence that the conjunctiva can be the source of human to human transmission and the route of entry of the virus into the body, its contagious nature has prompted recommendations for eye or facial protection when examining patients with conjunctivitis and those with COVID-19.10,11
Political Issues
The Philippine government’s policies enforcing social distancing and crowd avoidance; the public’s general adherence to community quarantine and lockdowns; and, the efforts of the private and public health sector are showing early signs of success in taming the COVID-19 epidemic. The government has begun to relax the restrictions in a bid to slowly revive the economy but is mainly depending on health statistics. If health parameters show worsening of the situation, the quarantine levels might be more restrictive again.
Legal Considerations
The Inter-Agency Task Force (IATF) published the guidelines on the implementation of community quarantine on April 29, 2020. Under Section 2.4.d., for areas under enhanced community quarantine (ECQ), “hospitals, medical, dental, and optometry clinics, pharmacies, and drug stores” are “allowed to work or operate with a skeleton (sic) workforce.” Less restrictions are imposed on professional services in areas under general community quarantine (GCQ) in Section 3.12
Executive Order No.112 imposed the recommendation of the IATF for the Management of Emerging Infectious Diseases (IATF-MEID) to extend the ECQ to May 15 in Metro Manila and other high-risk regions. Low-risk and medium-risk areas will be under GCQ.13,14 After that issuance, the executive orders have given way to media pronouncements by the Office of the President declaring GCQ, then after about 6 weeks back to modified ECQ (MECQ) and, back again to GCQ after 2 weeks in Metro Manila. The wearing of masks and face shield have become mandatory as are limited gatherings and physical distancing in public places. The Department of Trade and Industry (DTI) and the Department of Labor and Employment (DOLE) enforce the use of screening procedures and the collection of information for contact tracing in the workplace for all employees and customers.15 The health checklist is available on the DOLE website.
Economic Concerns
Gross domestic product (GDP) growth rate was 6.4% in the fourth quarter of 2019, bringing the full-year GDP growth to 5.9% for 2019.16 Because of the COVID-19 pandemic, negative growth or recession is expected for 2020 with recovery hopefully in 2021.
All sectors have been affected by the lockdown and quarantine. Manufacturing and agri-business might be operational sooner but demand will probably be depressed for some time.
Consumer-based services including health care will depend on lifting of both the quarantine and the suspension of public transportation. Income in ophthalmic practice is driven by outpatient consultations and is therefore contingent on the freedom of patients to move about. The throughput in patient consultations will also depend on the implemented safety measures that will prevent COVID-19 transmission, and the confidence and security of the patients in leaving their homes to travel to the clinics. Elective operations such as cataract surgery and refractive laser vision correction will depend in turn on the number of consultations.
There is a growing bias against hospital-based care with many patients expressing concern and fear about visiting ophthalmologists in hospitals despite reassurances by hospital management.
Technological Factors
Close patient contact is the norm when examining patients in ophthalmology. Telemedicine may be used as a substitute for face-to-face consultation but will probably leave both the doctor and the patient unsatisfied by the encounter since most ocular examinations involve the use of equipment.
Physical distancing, disinfection and regular sanitation of surfaces, frequent hand washing, the use of masks and gloves, and other measures will make it safe for patients to see the ophthalmologist. However, there will be examinations that require proximity and the handling of the eyelids. Risk can be mitigated by the use of gloves or cotton applicators and breath shields. Many diagnostic machines have automated functions that require less intervention by the technician performing the examination. Robotic features will reduce technician dependence even more, so that patients can be instructed at a distance or through a transparent barrier to perform the test with verbal assistance alone. Test results can be incorporated as soft copies in patients’ electronic records eliminating the need to pass printed copies by hand.
Environmental Concerns
The transmission of the virus through contaminated biological waste should not be anymore than that from ordinary trash since symptomatic COVID-19 patients will probably not be allowed in eye clinics. Most clinics are required to segregate biological waste in special containers to be treated by accredited medical waste disposal contractors.
The ecological impact of disinfectants and bleaching agents will increase. The increase in medical waste from discarded personal protective equipment (PPE) like gowns and masks is expected and requires proper disposal. A balance has to be achieved to protect the patients and the staff without overdoing it so as to not unduly harm the community and the environment.
RECOMMENDATIONS
The recommendations below embody the basic principle of the less contact with others, the better. The virus spreads by droplets emitted by a carrier who talks loudly, sings, sneezes, coughs, and otherwise expels mucus from the nasal passages and the oropharynx. The portal of entry of SARS-CoV-2 is also through the nose and mouth. The presence of the significant amounts of the virus in the conjunctiva and ocular surface is questionable, and, so is the possibility that the eyes can be the portal through which the virus infects the body.
Physical distancing of at least 1 meter with greater protection the farther the distance. Masks have a protective effect, more so with the N95 and surgical masks than the single layer cloth masks. Eye protection may have additional benefits but with low certainty.17
Transmission of the virus through fomites and aerosolized particles remains a concern especially in areas with a concentration of COVID-19 patients like hospital intensive care units where the more severe and presumably those with greater viral loads end up. SARS-CoV-2 viral particle detection by RT-PCR from surfaces within 1-5 meter from where 31 asymptomatic patients or companions sat was positive only in 2 samples in the 1 meter zone (1 on the slit lamp breath shield and 1 on the phoropter).18 The infectivity of those viral particles was not established. Sanitation and sterilization of commonly touched surfaces and frequent hand washing are therefore recommended despite the absence of evidence of viral transmission in real-life situations.19 Masks may have the additional benefit of deterring a person from unconsciously touching the mouth and nose. Improving room circulation by allowing fresh air to enter and avoiding the use of air conditioners have been advocated but may be impractical in a tropical environment. Air purifiers with high-efficiency particulate-absorbing (HEPA) filters and ultraviolet-light (UV) disinfection may serve the purpose of reducing contamination through aerosolized particles in a room with asymptomatic people.
To help screen yourself, employees, and patients and to facilitate contact tracing, the IATF requires the filling-up of a health checklist and temperature check.12 The effectiveness of screening efforts to identify symptomatic patients is dependent on strict implementation of guidelines for refusing to see those patients suspected to have COVID-19 or are probable carriers.
Since the lockdown, the Philippine Society of Cataract and Refractive Surgery (PSCRS) has anticipated the need for guidelines to help ophthalmologists in formulating their personalized policies and procedures when they resume practice. The evidence from more recent studies have been reviewed for the purpose of this article to update our guidelines. We also recommend the distillation of information and recommendations from other guidelines in formulating your own.20,21,22,23,24
Specific Recommendations for the Clinics
1. Consultation Hours
1.1. Shorten clinic hours and prioritize the more urgent and emergent cases.
1.2. Consider a telemedicine system for routine or non-urgent consultations.
1.3. Practice a strict by-appointment system so that there will be a good interval between patients and less likelihood of crowding in waiting areas. Do not accept walk-in consultations without proper screening.
1.4. All patients must wear a face mask at all times. Provide masks for those without one.
1.5. Only one relative should accompany the patient if the patient cannot come alone. Preferably, only the patient will enter the examination room.
1.6. All those who have to enter the clinics should be checked prior to entry for fever with a non-contact thermometer, should apply sanitizer to their hands, and step on a rug immersed in disinfectant. Using thermal scanners is non-invasive and fast but can sometimes be influenced by ambient temperature to which the patient has been recently exposed. An alternative valid target for temperature scanning is the wrist or inner forearm. Wheelchairs must also be wiped with a disinfectant prior to entry.
1.7. Maximize the gap between follow-up consultations with instructions on what to do or how to contact you or your clinic personnel especially if the next appointment becomes impossible.
1.8. If feasible, send a certificate that the patient has an appointment with you for their convenience when traveling to your clinic.
1.9. Interview for the purpose of screening patients by phone. Discourage patients who have anosmia, fever, headache, diarrhea, sore throat, and other flu-like symptoms, are sneezing or coughing, with a recent history of travel, with exposure to members of a household with recent travel, from coming to the clinic unless absolutely necessary or unless they have tested negative for the virus.
1.9.1. For patients who have had contact with a COVID-positive (COVID+) patient but are asymptomatic for the disease and have not been tested, a quarantine period of 14 days from the date of contact should be given prior to being given an appointment depending on your clinical judgement of their eye problem.
1.9.2. For COVID+ patients, dedicate a special time when there will be no other patients especially at end of the day, or, do virtual consultation by phone or video call for the meantime. Alternatively, arrange a home visit if an examination is required using adequate PPE.
1.10. Have a printed set of house rules for each patient on your procedures to prevent infection. If feasible, have one of your staff demonstrate and reiterate the safety procedures periodically.
1.11. Ban medical representatives and other non-essential personnel from entering the premises.
2. Staffing
2.1. If you have more than one or two assistants, stagger the schedules on a weekly or bi-weekly basis.
2.2. Provide orientation seminars on preventive measures in the clinic, when using public transportation and at home.
2.3. Everyone should change to a clean uniform and shoes upon arrival at the clinic. Change back to street clothes and shoes at the end of the day. Alternatively, a clean body gown and other PPE may be donned on arrival and put in a bag at the end of the day for disposal or to be laundered if reusable.
2.4. Hand washing and donning a clean surgical mask has to become routine at the beginning of the day. Ensure that you have adequate stocks without hoarding.
2.5. N95 masks, eye protection or other facial shields must be worn at all times especially for doctors and nurses who cannot avoid close contact with patients.
2.6. Disposable PPE must be disposed of in designated bins and handled as contaminated biological waste.
2.7. Whenever possible, use disposable cotton-tipped applicators to handle the eyelids. Require everyone to wash their hands with soap after physical contact with patients. Sanitize with compounds containing 70% ethyl alcohol or another sterilizing agent regularly and in between patients.
2.8. Discourage congregating for breaks or for meals. Breaks should be staggered and done in a place that is large enough for physical distancing and is well-ventilated.
2.9. Ask staff who have anosmia, fever, cough, headache and diarrhea to stay home, and get tested or self- quarantine for 14 days from the onset of symptoms. The same should be followed for those with definite exposure to COVID+ patients or suspects.
2.10. Provide hazard pay.
3. Physical Arrangement
3.1. Space between waiting chairs should be at least 1.5 meters apart and not be facing each other.
3.2. Barriers can be placed between patients and staff when practical.
3.3. Discourage patients from loitering around the premises.
3.4. Provide sanitizer/alcohol dispensers all around the premises within easy reach of the patients.
3.5. Provide a physical barrier (acrylic, glass, plastic sheets) between you and the patient when possible such as a breath shield at the slit-lamp and a maintain a distance of at least 1 to 2 meters when not examining the patient.
4. Clinic Procedures
4.1. Take the patient’s temperature as part of routine pre-examination procedures. Use your judgement in treating patients with fever; they can be asked to return after 2 weeks or they can be examined taking extreme precautionary measures.
4.2. Routine examinations like blood pressure and heart rate determination might be dispensed with temporarily if it has been part of your routine.
4.3. Avoid touching the patient unless absolutely necessary. Shaking hands, patting on the back and other habitual gestures should be consciously stopped.
4.4. Streamline your processes to minimize contact and time spent by the patients in your clinic. Use your clinical judgement on what routine tests can be eliminated without prejudice to the quality of care that you deliver.
4.4.1. Using a condensing lens in the slit-lamp may be safer than direct funduscopy.
4.4.2. Non-contact tonometers would be preferable for routine intraocular pressure (IOP) checking although air-puff tonometers may aerosolize the virus from the conjunctiva. Otherwise, eliminate tonometry if the IOP is not relevant to the patient’s problem. If IOP has to be checked by applanation, it is advisable to use disposable tonometer tips or protective sheathing. Otherwise, regular tonometer tips must be soaked in a 1:10 dilution bleach after each use. Sterilize the tonometer prism before and after examination.
4.4.3. Follow specific instructions for sanitizing and sterilizing instruments as recommended by manufacturers or distributors. Some manufacturers have provided recommendations below while others are referred to their user manuals for their respective instruments.
4.4.3.1. Zeiss Philippines provided answers to Frequently Asked Questions:
4.4.3.1.1. Can the HFA bowl be sterilized and how often? The HFA bowl is a specialized surface which is an integral part of the calibrated instrument. Therefore, it is not intended to be regularly cleaned but only if necessary due to possible contamination. It is critical that customers follow the procedure in the manual, to ensure the device is not damaged.
4.4.3.1.2. Can the OCT lenses be sterilized? No. Lenses should be cleaned with water, isopropyl alcohol, and acetone according to section 11.3.4 (Cleaning Heavy Contamination) of the CIRRUS manual.
4.4.3.1.3. What solvents can be used to clean ZEISS device surfaces? ZEISS recommends a disinfectant such as isopropyl alcohol (isopropyl alcohol contains 70% alcohol which is recommended by the Centers for Disease Control and Prevention [CDC]). Please refer to the user manuals for a list of appropriate other agents to use.
4.4.3.2. Medilight recommends dis- infection using any disinfectant currently used in the operating room, using a damp cloth and avoiding dripping of any fluids into the power supply or optical parts. For optical parts, use 1-2 drops of 99% ethanol on non-woven cloth and wipe from the center outward. Use dry non-woven cloth to totally dry the optical parts.
4.5. Don gloves if you have to touch the patient. Discard them after the examination before touching anything else. Follow your hospital or clinic procedures in the disposal of medical/biological waste.
4.6. When instilling drops, it is now more important than ever to follow recommendations to prevent contamination of the bottle.
4.6.1. Do not let the tip of the bottle touch the patient’s lids or lashes.
4.6.2. Cover the tip immediately.
4.6.3. The person instilling the drops must don clean gloves for each patient.
4.6.4. Disinfect the multi-dose bottles with 1:100 dilution bleach wipes after use. Store in a segregated area such as a tray lined with a cloth soaked in a nontoxic sterilizing solution. Do not leave them on your desk and examination table where they can be contaminated.
4.7. Avoid cash payments, if feasible. The person handling payments must sanitize their hands after taking cash or processing credit card payments before touching anything else
4.7.1. Cash should be stored in a closed container and handled like contaminated material.
4.7.2. Contactless, cashless transactions using electronic payments are advised.
5. Deliveries
5.1. The delivery person should not enter clinic premises. Care must be taken in handling deliveries. There are reports that the virus can remain viable in paper and cardboard for 24 hours up to 5 days and on metal and plastic surfaces for 2 to 3 days.
5.2. The deliveries must be placed on a table or area outside of the clinic, wiped down with an antiseptic solution by the person accepting the delivery, and external packaging removed and disposed of, when possible. Only the actual product is brought in and wiped down with antiseptic solution.
5.3. Ask suppliers about their procedures for keeping medical and surgical supplies free from contamination. Double packaging would be preferable so that the external packaging may be left outside of clinic premises.
5.4. Papers and all items must be handled by double-gloved hands. Outer pair must be removed carefully after the external package is removed or sanitized. The inner pair of gloves will be used when handling the actual product.
5.5. Items sold to patients such as medicines and spectacles should be wiped with antiseptic solution before passing on to the patient.
5.6. Delivery receipts or invoices accompanying deliveries should be transmitted electronically unless unavoidable. The papers must be set aside and kept in a container for as long as possible without touching them.
6. Cleaning and Sanitation
6.1. All furniture and fixtures (i.e. doorknobs, lightswitches, keyboards and faucets )must be wiped with a disinfectant solution regularly and periodically.
6.2. The examination chair, slit-lamp, clinic desk, and surfaces touched by the patient’s companion should be sprayed and wiped with disinfectant solution in between patients.
6.2.1. For non-disposable instruments, wipe thoroughly with 1:100 bleach dilution or bactericidal solution after each examination.
6.2.2. For slit-lamp and similar machines, disinfect forehead strap, chin rest, table surfaces, and all other surfaces that the patient may have touched Philippine Academy of Ophthalmology during the examination preferably by using a 1:100 dilution bleach wipe or 70% isopropyl/ethyl alcohol.
6.3. Walls and floors must be sanitized at the end of the day.
6.4. Bleach solutions and other disinfectants should be prepared daily and the remaining solutions discarded at the end of the day.
Specific Recommendations for Ocular Procedures and Surgery
In General
In consideration of the aforementioned issues and concerns, if a health facility and its operating room follow any of the published guidelines, elective eye surgeries not requiring general anesthesia can be performed on an outpatient basis.
Surgeries of patients with symptoms that may be attributable to COVID-19, those who have had exposure to confirmed COVID-19 cases, and those who are COVID positive should be postponed.
Patients for surgery should also be wearing masks and their temperatures taken routinely. Vital signs monitors should have disposable attachments that can be changed for each patient or have reusable attachments that are easy to sterilize between patients.
Draping creates an effective barrier between the patient and the staff. More assiduous care must be taken in the handling and disposal of nasal cannulas, breathing masks, and oxygen delivery systems if they are used.
Aseptic techniques and antisepsis protocols in the operating room should suffice in reducing the risk of transmission of the virus. Patient and surgical gowns are used only once.
Conjunctival wash with 5% povidone iodine should be used to clear the conjunctiva of the SARS-CoV-2 as studies with the SARS-CoV, a structurally similar virus, have shown25,26; although surgery of a patient with frank signs of conjunctivitis must be postponed. It has been suggested that povidone-iodine nasal spray and mouthwash may reduce cross infection.27
In short, regular operating room protocols paired with the clinic COVID-19 precautions already instituted should be enough to create a safe environment for most ophthalmic procedures.
If general anesthesia is required, because of the risk of aerosolizing nasopharyngeal flora and generating more secretions, a COVID-19 negative test should probably be part of the medical clearance prior to surgery. Nevertheless, for general anesthesia procedures, the facility should be capable of following the guidelines from the Philippine Society of Anesthesiologists.
For Cataract Surgery
There is no expected increase in morbidity in patients without conjunctivitis. There is still no evidence that ocular morbidity will increase should a patient with COVID conjunctivitis inadvertently undergo cataract surgery.
There should be no need to change pre-operative, intraoperative and post-operative procedures except for the extra care in ensuring that multi-dose bottles do not cross-contaminate patients when applying dilating and anesthetic drops.
Spacing between surgeries should allow adequate time for sanitation of the surgical chairs, microscopes, and other equipment between cases, and to avoid crowding in waiting rooms. There should be no problem with high-volume cataract surgeries if time permits.
For Refractive LASER Surgery
The only change necessary in refractive LASER cases, aside from spacing patients and sanitation procedures, is draping the face to create a barrier between the patient’s airway and the staff. Povidone-iodine application to the ocular surface may also be a prudent prophylactic measure to kill any viral infectivity of aerosolized particles during the procedure.
SUMMARY
Elective eye surgeries may safely resume if the additional precautions in the clinics and in the operating rooms are taken and as long as they are performed in patients with no COVID-19-like symptoms, under local anesthesia on an outpatient basis. These recommendations can serve as minimum safety standards that any surgeon or institution can build on or simplify depending on local conditions including the community prevalence of COVID-19 and transmission rates.28
Patients under general anesthesia will require special precautions related to intubation and manipulation of the airways. Full PPE for the staff in the room, ventilation control and air filtration, and stringent measures recommended by the Philippine Society of Anesthesiologists or your anesthesiologist will have to be followed. The addition of SARS-CoV-2 testing in the patient’s preoperative medical clearance would be a wise choice but a negative result should not reduce the need for precautionary measures.
There is no evidence that the conjunctival surface and tears if aerosolized during phacoemulsification or laser treatment can infect the surgeon or staff. It is reasonable to expect that povidone-iodine antisepsis will be virucidal in the highly unlikely event that a patient with the SARS-CoV-2 in the conjunctiva ends up on the operating table.
Surgical volume will depend on the capacity of the facility to space patients to prevent crowding in waiting areas and to have time for the staff to sterilize and sanitize the operating room equipment and surroundings between patients. Scheduling will probably be driven mostly by the balance between perceived urgency of the procedure and the degree of risk adversity of both the patient and the surgeon until good scientific studies emerge.
Recommendations on Testing
COVID-19 Diagnostic Tests
There are two tests for SARS-CoV-2 that have received the most attention: (1) the polymerase chain reaction (PCR) and (2) rapid antibody testing. These tests are not equivalent.
The PCR test, more formally known as the reverse transcription-PCR (RT-PCR), detects viral RNA but the process follows a series of steps involving among other things the conversion of RNA to DNA and its replication with alternating heating and cooling of the aliquot (amplification) so as to achieve the amount (termed “limit of detection” or LOD) that can be detected by the machine.29 This process alone can take 3 hours or more.
Proper handling of the specimen is crucial. The virus material is acquired by a nasopharyngeal or oropharyngeal swab; there are reports of a difference in yields using both methods favoring the former.30 The fragility of RNA and the need for the specimen to contain enough nucleic acid material to reach LOD can also account for reported false negative cases.31 It is not surprising that radiologists believe that pulmonary CT-scan can often be more sensitive in diagnosing COVID-19 pneumonia than RT-PCR. A retrospective study of 36 symptomatic patients showed 97.2% sensitivity for CT compared to 83.3% for the initial PCR test. Some patients who were initially PCR negative turned positive in the next test done after 2 days from admission and a few became PCR positive only on the third day.32 This implies that viral shedding is not consistently detected depending on the time the specimens are taken along the course of the disease.33
Protective equipment and biomedical cabinets prevent cross contamination of the specimen and infection of the medical technicians. To prevent any errors, labeling and cross-checking of patient information is done repeatedly especially since specimens have to be transported to accredited laboratories, of which there are only 30 nationwide. In most labs, the process of labeling, indexing, and transmittal of the patient’s information are performed manually using paper forms. The DOH requirement that all patient information, including history of exposure and a list of contacts with contact numbers, be encoded in an MS-Excel spreadsheet and sent to the DOH by the end of the day exacerbates the delay in the release of the results. Thus, tested individuals have to wait 2-3 days for their PCR test results.
The WHO and most Western countries are advocating mass testing as a prerequisite to containing the virus, although the former offers alternative strategies should testing capacity be overwhelmed.34 The emphasis of the media on the importance of testing belies the underlying complexity of the strategy that would make wide-spread testing a rational approach. Testing could uncover the extent of the disease in the community if the asymptomatic are also tested. Once a COVID-19-positive individual is identified, that person has to be quarantined and all his contacts within the past 2 weeks have to be traced and tested. Contacts would have to be tested too and those who are positive or who are symptomatic are treated like the index patient. Their contacts are traced as well until the cluster of individuals involved are all accounted for, tested, and managed accordingly. Such a strategy requires enormous resources and manpower aside from testing capacity.
Cognizant of our limited capacity and resources, the DOH has maintained that PCR testing should be limited to patients who are symptomatic of COVID-19, individuals who are immunocompromised and at risk of contracting the disease, those who have a history of contact with a confirmed COVID-19 case, and, healthcare workers involved in the management of COVID-19 patients. Individuals who fear they have the infection have to be first assessed by a licensed health professional if the test is indicated. The targeted testing capacity of the DOH is 30,000 per day by May 30, 2020.35
Antibody testing, on the other hand, is supposed to detect the presence of Immunoglobulins M (IgM) and G (IgG) that have formed in response to the SARS-Cov-2. These antibodies are generated days or weeks after the infection and the level of the antibody response is affected by the strength of the patient’s immune system, and, therefore, can only be used complementarily to PCR testing in the diagnosis and management of COVID-19 patients especially for research and epidemiologic studies.36 IgM is produced by the body earlier from the onset of the infection with the body’s first line of defense while IgG at a later phase. If IgM antibodies are detected, it is a possible indication of recent COVID-19 exposure. A PCR test would be needed to confirm if the infection is active. If IgG antibodies are detected, it is an indication of a past infection and might suggest that the person has gained some immunity to the virus.37 Doubts exist on specificity and sensitivity of rapid antibody serologic tests reported in many publications because they have not been peer-reviewed and are usually authored by the test development teams. The Health Technology Assessment (HTA) study conducted by the DOH has declared that antibody testing procedure is not recommended as a sole screening and diagnostic tool for COVID-19.38 The DOH itself maintains that antibody testing is not the recommended screening procedure39 and that it should not be used. The FDA warns against the online sale of test kits40 and reiterates that they are strictly for medical professional use.41
Roche, a biotech company based in Switzerland, has developed Elecsys® anti-SARS-CoV-2 antibody test that has been issued an Emergency Use Authorization (EUA) by the United States Food and Drug Administartion (US-FDA), and it is listed as one of the approved rapid immunoassay serologic test kits approved by the Philippine FDA as of May 19, 2020.42 Elecsys® has a reported specificity of 99.81% and an expected sensitivity of 100% 14 days after a COVID infection.43 The EUA, as the name implies, is a temporary approval for commercial availability of the test despite the fact that it has not gone through the rigorous testing procedures that the US FDA generally requires. The US FDA grants the EUA based on four criteria: (a) serious or life-threatening disease or condition; (b) evidence of effectiveness; (c) known and potential benefits outweigh known and potential risks; and, (d) there are no alternatives or if the alternatives are insufficient or inadequate.44 It shall expire when the COVID-19 crisis abates and by then real-life testing would have provided the data to determine the validity of the test’s sensitivity and specificity. An additional advantage of the Elecsys® test kit is that it can be run with the cobas® e analyzers, Roche’s fully-automated laboratory equipment, and churn out results in about 18 minutes with a throughput of up to 300 tests/hour.11
Regulations on Testing
There are no mandatory testing requirements issued by the government. WHO guidelines are recommendations and must be contextualized by national governments. The DOH is ramping up testing capacity but follows a strategy of prioritizing laboratory testing, lately expanding the prioritized group to include patients or healthcare workers with mild symptoms, relevant history of travel or exposure, and considered vulnerable, such as the elderly with co-morbidities, the immunocompromised, and those with high-risk pregnancies.45
The test that the DOH accepts as the gold standard for screening is the PCR method and it has authorized several government and private institutions as testing centers. It has reiterated that licensed health professionals have to order testing. While it recognizes the importance of COVID-19 testing as an important component of the response against the epidemic, it remains the option of the employers to conduct testing on asymptomatic employees upon returning to work.46
The DOH does not recommend antibody testing as a sole screening procedure for COVID-19. It has declared that the HTA study does not recommend the reimbursement of the antibody test by PhilHealth. However, it does not forbid the use of such tests as long as the results are properly interpreted by a physician and acted on accordingly including PCR testing for confirmation. The excerpt from the DOH Interim Guidelines on the Return-to-Work Section D17 regarding testing is reproduced below:
“Testing of Asymptomatic Returning Employees
1. While testing is an important component of response against COVID-19, limitations on their reliability and validity shall be recognized.
2. Employers who opt to conduct testing may do so in a representative sample of those who have returned to work physically and have a high risk of contracting COVID-19 due to the nature of the work (e.g. frontliners).
3. Testing using RT-PCR among representative samples for baseline can be conducted to look for any evidence of asymptomatic transmitters.
a. If tested positive, the returning employee/worker is a COVID-19 case and will be isolated and referred accordingly for appropriate management. All close contacts shall be isolated and tested accordingly based on Department.
b. If found negative, returning employee and worker can continue working with usual precautions.
c. If initially tested negative but developed symptoms, the employee must be tested accordingly based on Department Memorandum 2020-0180.
i. If found positive, all close contacts of returning employees and workers shall be isolated and tested accordingly based on Department Memorandum 2020-0180.
d. Employers shall report the results to DOH in accordance with Administrative Order No. 2020-0013, entitled “Revised Guidelines for the Inclusion of COVID-19 in the List of Notifiable Diseases for Mandatory Reporting to the Department of Health” and Administrative Order No. 2020-0014 entitled “Guidelines in Securing a License to Operate a CoVID-19 Testing Laboratory in the Philippines.”
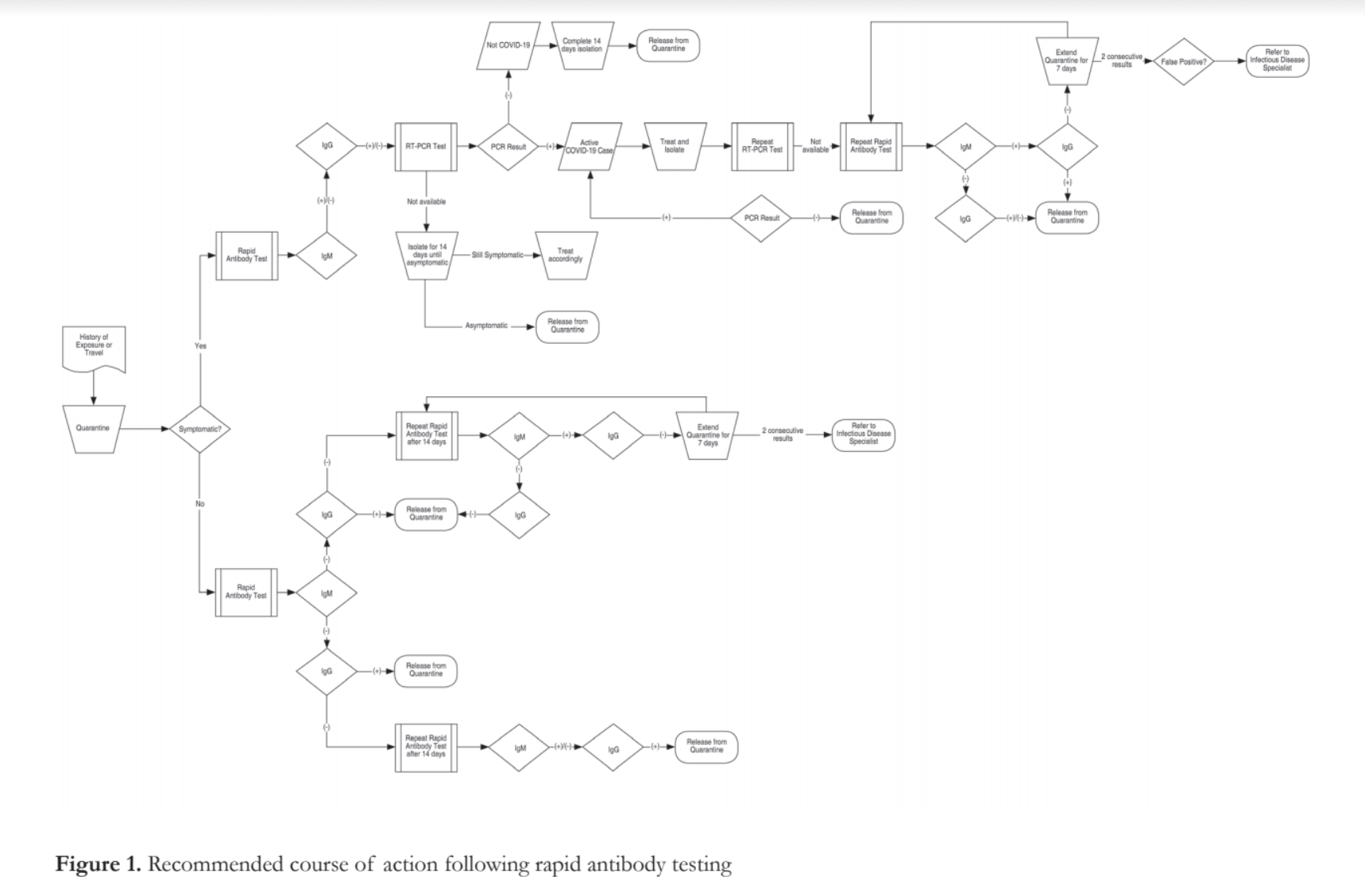
4. Alternatively, testing using FDA-approved rapid antibody-based tests representative samples for baseline can also be conducted up to every 14 days.
a. Employees who test IgM negative and IgG negative, or IgG positive regardless of IgM results may continue to work.
b. Employees who test IgM positive but IgG negative on the 1st test shall be isolated for 14 days and repeat testing on the 14th day. If results are still IgM positive and IgG negative, extend quarantine by seven-day increments and repeat testing. If persistently IgM positive but IgG negative for two consecutive re-testings after the 1st 14 day-period, consider potential false positives and confer with infectious diseases specialists.
c. Employers shall submit to hrtucovid19results@gmail.com the results of the rapid antibody tests among the sample representatives using the format available on https://bit.ly/RDTReportingForm.
5. Cost of the test not covered by PhilHealth shall be borne by the employer.”
A DOH circular47 provides a table in its annex with the recommended courses of action depending on rapid antibody test results which has been translated diagrammatically in Figure 1.
The DOH, DOLE, and DTI do require safety precautions in the workplace including proper ventilation, work at home arrangements if possible, physical distancing, sanitation and procedures promoting hygiene, masks and other PPEs for frontliners depending on the nature of their work, and daily screening protocols to check for symptoms, for fever, and history of exposure to COVID-19 cases or travel within the past 14 days. A sample algorithm and questionnaire to systematize the daily screening of employees is found in the annex of the DTI and DOLE Interim Guidelines on Workplace Prevention and Control of COVID-19.15 Testing is not mandated in the same guidelines.
Testing Prior to Cataract Surgery and Minor Elective Ophthalmic Procedures
Assuming that eye clinics follow the general recommendations of various agencies and specific guidelines of the PAO, the PSCRS, or other reputable ophthalmological organizations, ophthalmologists should be limiting their patients to urgent cases and to those who do not exhibit any symptoms associated with COVID-19, have no history of exposure to a confirmed COVID-19 case or have not traveled to a COVID-19 hot spot within the last 14 days. They are presumably carrying out safety precautions in the conduct of their clinics. Hence, those who will be scheduled for elective surgery should have also been screened for COVID-19 symptoms and examined with the safety precautions in place.
Definitely, the development of symptoms or exposure in a patient before surgery should cause the postponement of the case until a proper assessment is made and a quarantine period is observed. PCR testing is indicated for these cases under DOH regulations to confirm the diagnosis.
In patients who pass the initial screening procedures, without a PCR test, there is no way of knowing whether they are COVID-19 asymptomatic carriers.
If a patient is not a carrier, then, he or she does not pose a risk to the surgeon, the anesthesiologist, and the other healthcare personnel. On the contrary, it is the doctor and the staff who pose a threat to the patient if they are asymptomatic carriers. Doing a PCR test on all the doctors and the staff is certainly an option, however, it may not be practical and sustainable. A positive PCR test is helpful and will cause a ban on the doctor or employee from working in the clinic, put that person in quarantine, and refer for further management if symptoms surface. It will mean that all contacts of the individual will have to be traced and tested including all clinic personnel and the patients exposed to that person. Effectively, the clinic would have to be shutdown to avoid risking transmission to patients and other personnel. A negative PCR test result, false negatives aside, can change within days if the tested individual is exposed to a COVID patient after the specimen is taken. To be useful, the test will have to be repeated periodically. In both scenarios, the expense would be substantial since a single PCR test costs about PhP4,000. If the ultimate objective is to protect the patient while rendering the required service, implementing physical distancing, wearing masks and PPEs, and mandating precautionary measures like frequent hand washing, are probably more cost-effective in actively preventing the transmission of the virus assuming any of the healthcare workers are asymptomatic carriers, without depriving the patient of the service.
If the patient is a carrier, he or she poses a threat to the staff. The risk that an asymptomatic carrier sheds the virus is thought to be likely because of the high incidence of community transmission although a study of an asymptomatic carrier and all his contacts suggests that this is not always the case.48 The risk is mitigated by the precautions already instituted by the healthcare facility. In the operating room, where infection control measures are even more stringent, the risk to the staff should be lower except for the close contact during the procedure and the possibility of aerosolization of nasal or oral secretions especially during intubation and extubation. The anesthesiologists feel particularly vulnerable in this situation and the Philippine Society of Anesthesiologist wants testing all patients prior to surgery to be considered but testing “should be determined by patient indications and procedure needs.”
The PSCRS recommends that the patients and all the operating room staff wear N95 masks. The masks plus the barrier drapes should prevent staff and patient exposure to aerosolized nasal and oral secretions for cases done under topical anesthesia, such as routine cataract surgery, pterygium excision, and minor surgical procedures, including intra-vitreal injections. The use of goggles or face shields may be considered but may impede good visualization without any probable benefit.
The risk of exposure from conjunctival secretions and aerosolization during phacoemulsification is probably negligible even in a COVID-19 asymptomatic carrier as pointed out in the updated PSCRS paper on the resumption of cataract and other elective ophthalmic surgical procedures. The relevant discussion is reproduced below:
“The coronavirus is highly transmissible and detected in tears and ocular surface but at very low rate.1 Although there are numerous case reports of conjunctivitis and ocular surface disease as part of the presenting symptoms of COVID-19, there are no other ocular morbidities that have been associated with it.4 A laboratory study where viral replication of SARS-CoV-2 was demonstrated in cultures of ex-vivo conjunctiva proposes that the conjunctiva is a route of entry of the virus6. In a published meta-analysis, the incidence of conjunctivitis was found to be 3% and 0.7% in severe and non-severe COVID-19 cases, respectively.7 In a large retrospective study of COVID-19 treated patients in Hubei Province, China, one third of patients manifested with conjunctivitis, although only 16.7% of confirmed COVID-19 patients by nasopharyngeal RT- PCR also had positive results from the conjunctival RT-PCR testing.8 A prospective study in Singapore on seventeen RT-PCR positive COVID-19 patients failed to show any evidence of the virus from systematic tear sampling even in the patients who exhibited conjunctival congestion and chemosis.9 Despite the scarcity of the evidence that the conjunctiva can be the source of human to human transmission and the portal of entry of the virus into the body, its contagious nature has prompted recommendations for eye or facial protection when examining patients with conjunctivitis and those with COVID-19.10,11
Furthermore, povidone-iodine conjunctival wash done routinely in patient preparation prior to eye surgery is probably enough to clear the ocular surface of the COVID-19 virus, if it is present at all, as studies with the SARS-CoV, a structurally similar virus, have shown.25,26”
Procedures, whether minor or not, that may require manipulation of the airways such as the introduction of laryngeal masks or intubation for general anesthesia or dacryocystorhinostomy especially with the use of high speed drills, are another matter because of the risk of aerosolization of nasopharyngeal and oropharyngeal secretions.
Rapid antibody testing may lead to a more confusing situation. Its only advantage is that these point-of-care tests come out with results very quickly. The disadvantage is that it is almost useless as a screening test because it takes at least a few days from exposure to the virus for the body to mount an antibody response, and the antibody response lingers way beyond the course of the infection. A negative result does not rule out the possibility that the tested individual is a COVID-19 carrier. A positive result for IgM antibodies will need a PCR test to determine if there is an active infection while a positive result for IgG will need a PCR test to determine if the infection is no longer active.
CONCLUSION
PCR COVID-19 testing prior to routine, elective cataract and minor ophthalmologic surgeries done under local anesthesia does not seem to be indicated in asymptomatic patients and healthcare workers including the surgeon as long as mandated screening procedures are performed and proper safety measures for infection prevention and control are implemented.
ACKNOWLEDGEMENT
This paper is a collaborative effort of the PSCRS core group to help dissect the issues on the mitigation of risk of COVID-19 transmission and on testing for COVID-19 infection in the Philippine setting as they pertain to clinic operations of a general ophthalmologist and to outpatient elective ophthalmic procedures in hospitals and ambulatory surgical centers. While the content was reviewed by a panel consisting of Drs. Reuben Aquino, Cesar Espiritu, Adel Samson, Ivo Dualan, Richard Kho, and Ellen Sy, any errors should only be attributable to the authors. The paper may appear normative in tone but it does not purport to convey the message that PSCRS is the ultimate authority on COVID-19 in ophthalmic practice. The reader is expected to critically analyze the views in this paper and other similar guidelines to determine the best course of action to take with due consideration to the peculiarities of his or her practice and any future regulations issued by the government. Furthermore, this paper per se should not be used to contravene policies and procedures promulgated by the health facilities where they practice.
REFERENCES
1 .Shang X, Chen X, Chen L, et al. The evidence of SARS-CoV-2 infection on the ocular surface. Ocul Surf. 2020;18:360-2.
2. Torres-Costa S, Lima-Fontes M, Falcao-Reid F, Falcao M. SARS-COV-2 in Ophthalmology: Current evidence and standards for clinical practice. Acta Med Port. 2020;33:593-600.
3. Amesty MA, Alio del Barrio JL, Alio JL. COVID-19 disease and Ophthalmology: An update. Ophthalmol Ther. 2020;9:415–426.
4. Hu K, Patel J, Swiston C, Patel BC , Ophthalmic manifestations of coronavirus (COVID-19). [Updated 2021 Feb 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK556093/
5. Gangaputra SS, Patel SN. Ocular symptoms among nonhospitalized patients who underwent COVID-19 testing. Ophthalmology. 2020.127:1425-7.
6. Hiu KPY, Cheung MC, Perera RAPM, et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-Ivo and in-vitro cultures. Lancet Respir Med. 2020;8(7):687-95.
7. Loffredo L, Pacella F, Pacella E, et al. Conjunctivitis and COVID-19: a meta-analysis. J Med Virol. 2020;92(9):1413-4.
8. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575-8.
9. Seah IYJ, Anderson DE, Kang AEZ, et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 2020;127:977-9.
10. Chen MJ, Chang KJ, Hsu CC, Lin PY, Liu CJ. Precaution and prevention of coronavirus disease 2019 (COVID-19) infection in the eye. J Chin Med Assoc. 2020;83:648-50.
11. Xia J, Tong J, Liu M, et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92(6):589-94.
12. Inter-Agency Task Force for the Management of Emerging Infectious Diseases. Omnibus Guidelines on the Implementation of Community Quarantine in the Philippines. April 29, 2020: https://www.officialgazette.gov.ph/downloads/2020/05may/20200429-Omnibus-Guidelines-on-the-Implementation-of-Community-Quarantine-in-the-Philippines.pdf (accessed May 22, 2020).
13. Duterte RD. Executive Order No. 112. April 30, 2020: https://www.officialgazette.gov.ph/downloads/2020/04apr/2020030-EO-112-RRD.pdf (accessed May 22, 2020).
14. Inter-Agency Task Force for the Management of Emerging Infectious Diseases. Resolution No.30. April 29, 2020: https://www.officialgazette.gov.ph/downloads/2020/04apr/20200429-IATF-RESOLUTION-NO-30-RRD.pdf (accessed May 22, 2020).
15. Lopez RM, Bello SH. DTI and DOLE Interim Guidelines on Workplace Prevention and Control of COVID-19. May 1, 2020: https://www.dole.gov.ph/news/dti-and-dole-interim-guidelines-on-workplace-prevention-and-control-of-covid-19/ (accessed May 22, 2020).
16. Philippine Statistics Authority. GDP posts 6.4 percent growth in the fourth quarter of 2019; 5.9 percent for full-year 2019. January 23, 2020. https://psa.gov.ph/content/gdp-posts-64-percent-growth-fourth-quarter-2019-59-percent-full-year-2019 (accessed May 4, 2020).
17. Chu DK, Akl EA. Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973-87.
18. Aytogan H, Ayintap E, Yilmaz NO, et al. Detection of coronavirus disease 2019 viral material on environmental surfaces of an ophthalmology examination room. JAMA Ophthalmol. 2020;138:990-993.
19. Goldman E. Exaggerated risk of transmission of COVID-19 by fomites. Lancet. 2020;20:892-893.
20. Nguyen AX, Gervasio KA, Wu AY. Differences in SARS-CoV-2 recommendations from major ophthalmology societies worldwide. BMJ Open Ophthalmol. 2020;5(1):e000525.
21. K khor WB, Yip L, Zhongshan P, et al. Evolving Practice Patterns in Singapore’s Public Sector Ophthalmology Centers During the COVID-19 Pandemic. Asia Pac J Ophthalmol (Phila). 2020;9:285-90.
22. Chung SSL, Wong CYK, Chan JCK, Chan CKM, et al. Ophthalmology in the time of COVID-19: experience from Hong Kong Eye Hospital. Int J Ophthalmol. 2020;13:851-859.
23. Reda AM, Ahmed WM. Standard precaution measurements during ophthalmology practice in the pandemic stage of COVID-19. Int J Ophthalmol. 2020;13:1017-1022.
24. Tang SWK, Romano MR, Wong DHT, et al. The use of personal protective equipment in clinical ophthalmology during corona virus disease-2019: a review of international guidelines and literature. Curr Opin Ophthalmol. 2020;31(5):435-446.
25. K kariwa H, Fuji N, Takeshima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology. 2006;212(SUPPL 1):119-123.
26. Eggers, M, Eickman M, Zorn J. Rapid and effective virucidal activity of povidone-iodine products against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Modified Vaccinia Virus Ankara (MVA). Infect Dis Ther. 2015;4(4):491-501.
27. Bayley JK, Sunkaraneni S, Challacombe S. The use of povidone iodine nasal spray and mouthwash during the current COVID-19 pandemic may reduce cross infection and protect healthcare workers. March 28, 2020: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3563092 (accessed May 4, 2020).
28. Sharma N, Khama P, Sachsen MS, et al. All India Ophthalmological Society – Preferred practice in refractive surgery during the COVID-19 pandemic. Indian J Ophthal. 2020;68(7):1263-8.
29. Stoppler MC. Polymerase chain reaction. https://www.medicinenet.com/pcr_polymerase_chain_reaction/article.htm#what_is_rt_pcr (accessed May 22, 2020).
30. Wang X, Tan L, Wang X, et al. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoC-2 detection in 353 patients received tests with both specimens simultaneously. Int J Infect Dis. 2020;94:107-9.
31. Feng H, Liu Y, Lv M, Zhong J. A case report of COVID-19 with false negative RT-PCR test: necessity of chest CT. Jpn J Radiol. 2020;38(5):409-410.
32. Long C, Xu H, Shen Q, et al. Diagnosis of the coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020;126:108961.
33. Kucirka LM, Lauer SA, Laeyeendecker O, et al. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020;173(4):262-7.
34. Word Health Organization. Laboratory testing strategy recommendations for COVID-19. Interim Guidance. March 21, 2020. https://apps.who.int/iris/bitstream/handle/10665/331509/WHO-COVID-19-lab_testing-2020.1-eng.pdf?sequence=1&isAllowed=y (accessed May 23, 2020).
35. Duque FT III. Minimum Health System Capacity Standards for COVID-19 Preparedness and Response Strategies. May 4, 2020. https://doh.gov.ph/sites/default/files/health-update/ao2020-0016.pdf (accessed May 23, 2020).
36. World Health Organization. Advice on the use of point-of-care immunodiagnostic tests for COVID-19. April 8, 2020. https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19 (accessed May 22, 2020).
37. Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020;92(9):1518-1524.
38. Duque FT III. Department Circular 2020-0184. April 9, 2020. https://doh.gov.ph/sites/default/files/health-update/dc2020-0184.pdf (accessed May 22, 2020).
39. Duque FT III. Department Circular 2020-0160. March 31, 2020. https://doh.gov.ph/sites/default/files/health-update/dc2020-0160.pdf (accessed May 22, 2020).
40. Food and Drug Administration. FDA Advisory 2020-498. April 1, 2020. https://www.fda.gov.ph/wp-content/uploads/2020/04/FDA-Advisory-No.2020-498.pdf (accessed May 22, 2020).
41. Food and Drug Administration. FDA Advisory 2020-497. April 1, 2020. https://www.fda.gov.ph/wp-content/uploads/2020/04/FDA-Advisory-No.2020-497-.pdf (accessed May 22, 2020).
42. Food and Drug Administration. FDA Advisory 2020-409. https://www.fda.gov.ph/fda-advisory-no-2020-409-list-of-approved-covid-19-test-kits-for-commercial-use/ (accessed May 22, 2020).
43. Food and Drug Administration. List of Approved Immunoassay Test Kits for Commercial Use. May 19, 2020. https://www.fda.gov.ph/fda-approved-7-additional-kits-2-pcr-5-serologic-as-of-19-may-2020-total-registered-covid-19-test-kits-for-commercial-use-is-now-93/ (May 22, 2020)
43. Roche Group Media Relations. Roche develops new serology test to detect COVID-19 antibodies. April 17, 2020. https://www.roche.com/media/releases/med-cor-2020-04-17.htm (accessed May 22, 2020)
44. US-Food and Drug Administration. Emergency Use Authorization of Medical Products and Related Authorities. January 2017. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/emergency-use-authorization-medical-products-and-related-authorities (accessed May 22, 2020).
45. Department Circular 2020-0179. April 9, 2020 https://doh.gov.ph/sites/default/files/health-update/dc2020-0174.pdf (accessed May 22, 2020).
46. Duque FT III. Department Memorandum 2020-0220. May 11, 2020. https://doh.gov.ph/sites/default/files/health-update/dm2020-0220.pdf (accessed May 22, 2020).
47. Duque FT III. Department Memorandum. 2020-0180. April 16, 2020. https://doh.gov.ph/sites/default/files/health-update/dm2020-0180.pdf (accessed May 22, 2020).
48. Gao M, Yang L, Chen X, et al. A study on infectivity of asymptomatic SARS-CoV-2 carriers. Respir Med. 2020;169:106026.

