Multicenter survey of badminton-related eye injuries
Katherine V. Zamora, MD , Harvey S. Uy, MD
ACUTE primary angle-closure glaucoma is a potentially blinding condition commonly regarded as an ophthalmic emergency. Initial medical therapy is aimed at lowering the intraocular pressure (IOP), and once reduced, laser peripheral iridotomy (LPI) is usually performed. LPI has been established as a safe and effective treatment for the affected and fellow eye. It has superseded surgical peripheral iridectomy as the definitive treatment of choice for acute primary angle closure (APAC). Its major advantage is that it is noninvasive and can be performed quickly and safely on an outpatient basis, without the attendant risks of invasive surgery like hemorrhage, wound leakage, and infection. Studies in Caucasian eyes have shown that LPI is effective in maintaining IOP control in the long term after APAC.1, 2 However, in a series of Asian eyes, only 41.8% were successfully treated with LPI alone.3 Many patients experienced a rise in IOP within 6 months despite a patent LPI.3, 4 It is believed that APAC tends to be more severe in Asian patients who often consulted late.3 These findings emphasize the need to monitor IOP carefully in the first few months after LPI to detect any subsequent IOP rise. Malaysia is a multiracial country composed largely of Malays, Chinese, and East Indians. Even though the incidence and long-term outcomes of APAC in Malaysia are not known, data on the effectiveness of LPI after an APAC attack will be useful in the management of these patients. Thus, we determined the percentage of eyes with APAC that developed raised IOP after definitive treatment with
LPI. We quantified the subsequent treatment needed to control the elevated IOP, and determined the factors influencing the final IOP outcome in a Malaysian population after an attack of APAC.
METHODOLOGY
Records of consecutive patients presenting with APAC at the Penang Hospital from March 1, 1996 to November 30, 2003 were reviewed. Included were patients presenting with APAC who have completed at least 6 months of followup, with or without glaucomatous optic neuropathy at presentation. Patients with less than 6 months of followup, those with chronic angle closure without symptoms of APAC, those with secondary angle closure, and those who have had previous intraocular surgery or filtration surgery in the affected eye were excluded. Acute primary angle closure1,5 is defined as the presence of at least 2 of the following symptoms: ocular or periocular pain, nausea and vomiting, and a history of intermittent blurring of vision with halos. The presenting IOP measured by Goldmann applanation tonometry must be more than 21 mm Hg with at least 3 of the following ocular signs: conjunctival injection, corneal epithelial edema, mid-dilated unreactive pupil, shallow anterior chamber, glaukomflecken, and iris atrophy. Gonioscopy must show
an occludable angle. Presenting IOP is the IOP at consultation as measured by Goldmann applanation tonometry. Duration of symptoms before presentation is the time symptoms first appeared to the time of consultation at the eye clinic. Symptoms included pain, redness of the eye, nausea and vomiting, and seeing haloes around light. Duration of less than 24 hours is taken as 0 day, less than 48 hours as 1 day, and so forth.
Time to control the IOP to ≤ 21 mm Hg after an attack of APAC is the period (in days) when patient presented with symptoms of APAC to the time IOP was reduced to 21 mm Hg or less. If the IOP was controlled in less than 24 hours, it was recorded as 0 day. If the IOP was controlled between 24 and 48 hours, it was recorded as 1 day, and so forth. Long-term raised IOP5 is defined as IOP greater than 21 mm Hg and requiring continuing treatment by medication or surgery despite LPI. Data collected included demographic characteristics, duration of symptoms before presentation, IOP and Snellen visual acuity at the time of diagnosis, time to control the IOP to ≤ 21 mm Hg after an attack of APAC, methods of treatment of the acute episode, associated medical problems, time to raised IOP after an attack of APAC, antiglaucoma treatment required including the time of commencing treatment and the number of topical medications needed, surgery required including
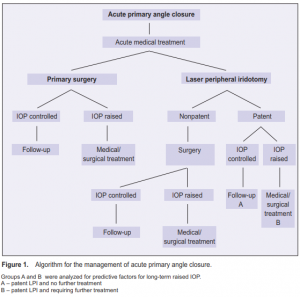
timing and type of surgery, and duration of follow-up. Figure 1 shows the algorithm for APAC management. Factors predictive of the development of subsequent increase in IOP requiring further treatment were analyzed. Significance level was ≤ 0.05.
RESULTS
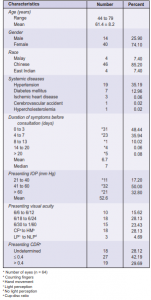
A total of 1,271 new glaucoma cases from March 1996 to November 2003 were reviewed. Of these, 54 consecutive patients (64 eyes) met the selection criteria. Ten patients (18.5%) presented with bilateral acute attacks. There were 35 left eyes (54.7%) and 29 right eyes (45.3%). The mean follow-up period was 30.9 ± 23.6 months (range 6 to 93 months, median 25.5 months). Forty-six (85.2%) patients were Chinese, 4 (7.4%) Malays,
and 4 (7.4%) East Indians. Forty (74.1%) were females with a mean age of 61.4 ± 7.8 (range 44 to 79) and 14 (25.9%) males with a mean age of 61.4 ± 9.6 years (range 45 to 78). Twenty-one patients (38.9%) had cardiovascular diseases, most common of which were hypertension (35.2%) and diabetes mellitus (13.0%). The mean duration of symptoms was 6.7 ± 9.1 days (range half an hour to 60 days) (Table 1). The mean IOP at presentation was 52.6 ± 14.1 mm Hg (range 26 to 80 mm Hg).
The initial medical treatment was similar in every case; it consisted of
intravenous 500 mg acetazolamide, followed by oral acetazolamide, topical pilocarpine, timolol, and betamethasone or dexamethasone. In some cases, intravenous mannitol or oral glycerol was also administered. One patient underwent laser iridoplasty during the acute management. Fifty-two eyes (81.3%) underwent sequential argon laser and Nd:YAG laser peripheral iridotomy. Out of these, 48 eyes (92.3%) had a patent
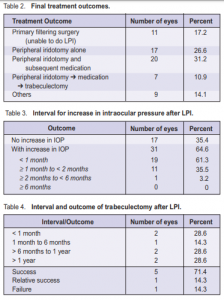
iridotomy. Out of the 4 eyes where LPI was not patent, 2 underwent trabeculectomy, one extracapsular cataract extraction, and one phacoemulsification. The mean time to performing LPI was 6.1 ±15.1 days. In 43 eyes (89.6%), LPI was performed within one week of the attack. LPI was not performed in 12 eyes (18.8%). One eye of a patient who consulted late was blind at presentation. Only medical treatment was given. Seven eyes (58.3%) underwent primary trabeculectomy, 3 (25.0%) underwent phacotrabeculectomy, and 1 (8.3%) had extracapsular cataract extraction combined with trabeculectomy. The final treatment outcomes are shown in Table 2. Of the 48 eyes that underwent successful LPI, only 17 had no subsequent IOP increase on follow-up; 31 developed a subsequent increase requiring further treatment. One patient, who presented 7 days after the acute attack with an IOP of 56 mm Hg and no light perception, had uncontrolled IOP despite patent LPI and antiglaucoma medications. Six needed 1 topical medication, 15 needed 2, and 9 needed 3 or more. Nineteen (61.3%) eyes were controlled with topical medications alone, while 11 (35.5%) eventually underwent trabeculectomy, lens extraction, or combined lens extraction and trabeculectomy to achieve IOP control. Thirty-one eyes had raised IOP within 6 months of the LPI (Table 3). The increase in IOP occurred in less than a month in 19 (61.3%) eyes and in less than 2 months in 30 (96.8%). Despite antiglaucoma medications (range 1 to 4), 7 eyes eventually underwent trabeculectomy to achieve IOP control (Table 2). The mean time to trabeculectomy after LPI was 5.6 months (range 0.3 to 15.7). Trabeculectomy was performed within 1 year of the LPI in 5 (71.4%) eyes. Five eyes had final IOP of 21 mm Hg or less without medication (success), 1 had final IOP of 21 mm Hg or less with medication and 5- fluorouracil needling of the encapsulated bleb (relative success), and 1 had final IOP of 21 mm Hg or less with repeat trabeculectomy and subsequent medication (failure of initial trabeculectomy) (Table 4). All 5 eyes with successful trabeculectomy underwent surgery between 2.5 and 15.7 months after LPI. The other 2 eyes with failed or partially successful trabeculectomy underwent surgery within 10 days of LPI. Eight possible predictive factors for the development of a subsequent increase in IOP requiring further treatment were analyzed (Table 5). These included age and sex of the
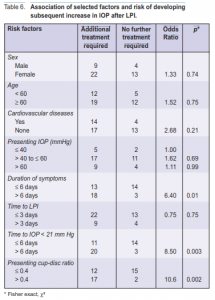
patient, duration of symptoms, presenting cup-disc ratio, time to control the IOP to ≤ 21 mm Hg (duration of attack), level of presenting IOP, time to perform LPI, and the presence of cardiovascular diseases. Only duration of symptoms, presenting cup-disc ratio, and duration of attack showed statistically significant differences betwee patients requiring long-term treatment or not. Patients who presented with symptoms 6 days or more after the attack had 6.4 times higher risk (p = 0.01). Patients who had their IOP controlled after 7 days had 8.5 times risk (p = 0.003) and those presenting with cup-disc ratio >0.4 had 10.6 times risk (p = 0.002) of requiring longterm treatment.
DISCUSSION
APAC is a major form of glaucoma in East Asia.6-8 It is common in Chinese and Sino-Mongolian populations.7-9 Considering that Penang has a predominantly Chinese population, this study found acute angle closure to be
with LPI alone and 64.6% developed subsequent increase in IOP, of which 70% needed additional 1 to 2 antiglaucoma medications. The most common medications used were pilocarpine, timolol, latanoprost, and dorzolamide. Eleven eyes eventually underwent trabeculectomy, lens extraction, or both to achieve further IOP control. The differences in the efficacy of LPI among the different studies may be the result of differences in study population, characteristics, and definitions of APAC. Series that included patients with preexisting glaucomatous optic neuropathy would most likely have a higher failure rate of LPI than series involving patients with normal optic disc. The appropriate treatment given during the acute attack is also not standardized and the type of treatment for long-term raised IOP depends largely on the clinician. Nevertheless, the success rate of LPI seems to be higher among Caucasians probably because they have better access to medical care, consulted earlier, and may have a milder form of the disease. Our study showed that patients who consulted more than 6 days after an attack of APAC and whose IOPs were controlled after 6 days were likely to require additional long-term treatment (p = 0.01 and 0.003 respectively). The
longer the attack remained, the greater was the likelihood of damage to the trabecular meshwork with subsequent development of peripheral anterior synechiae (PAS) that compromise the aqueous drainage. The long–term raised IOP usually developed within the first 2 months of attack (96.8%). All eyes developed raised IOP within 6 months of the LPI, illustrating that once angle damage had occurred, LPI was no longer effective in preventing a subsequent rise in IOP. Thus, all patients must be monitored closely for possible treatment within the first 6 months of attack, especially during the first 2 months. Seven eyes (10.9%) eventually underwent trabeculectomy to achieve IOP control. The mean time to trabeculectomy after LPI was 5.6 months. This illustrates the importance of follow-up in the first 6 months of those presenting with raised IOP to determine the need for further surgery. Our series also showed that early trabeculectomy during an acute attack was more likely to fail or be partially successful largely because these eyes may be markedly congested and have a more severe form of APAC with wide areas of PAS. In such instances, adequate medical treatment to include antiinflammatory agents during the acute stage is necessary to increase the success rate of trabeculectomy in APAC. Eight possible predictive factors for the development of a subsequent increase in IOP requiring treatment were analyzed. In this series, gender, age, presence of associated cardiovascular diseases, and presenting IOP did not increase the risk. These findings are similar to those reported by Aung et al. in a Singaporean population.3 Presenting symptoms of more than 6 days and duration of attack of more than 6 days showed statistically significant risks of the long-term need for treatment following LPI. Similar results reported by Saunders showed that duration of symptoms prior to presentation was significant in
distinguishing between patients who will be cured by simple iridotomy and those who will require additional
medication or surgery.15 Thus, it is important to educate the public on the symptoms of acute primary angle closure and the need to seek treatment immediately. This would help reduce morbidity and health costs associated with this potentially blinding condition. The primary-care physician should be educated in recognizing the condition early and to refer such cases without delay. A comprehensive treatment protocol should also be formulated so that a standardized treatment regimen can be given adequately. The time to performing LPI did not contribute to the need for long-term treatment in our study, suggesting that
immediate control of IOP by whatever means is the main contributing factor to the long-term outcome. Thus, medical treatment to abort the acute attack and bring down the IOP to a safe level may be effective in preventing damage to the trabecular meshwork and drainage angles during an acute episode. Eyes presenting with a cup-disc ratio of more than 0.4 was a significant contributing factor to long-term need for treatment (p = 0.002). A large cup-disc ratio may be an indication of a more serious attack with resultant glaucomatous optic neuropathy. In such instances, the clinician is required to bring down the IOP to a much lower level commensurate to the degree of glaucoma damage. This is in concordance with findings that LPI is not a satisfactory long-term therapy in eyes with established primary angle-closure glaucoma with glaucomatous optic neuropathy and visual-field damage.18, 19 The drawback of this study is that it is retrospective with
variable follow-up. Several ophthalmologists and medical officers were involved in the care of the patients. The data obtained from hospital case notes were not collected for purposes of research and were frequently inadequate. Detailed and standardized gonioscopy was also lacking. A prospective study of this sort would require several years and may be needed in spite of the attendant costs and administrative difficulties. In summary, LPI alone was found to be insufficient in maintaining IOP less than 22 mm Hg after an attack of APAC in two-thirds of the patients in this series. They developed elevated IOP within 6 months of LPI. Close monitoring of patients within the first 6 months of APAC is, therefore, important to detect those requiring further medical or surgical treatment. Predictive factors on the long-term need for additional treatment include presenting symptoms of more than 6 days, duration of attack of more than 6 days, and a presenting cup-disc ratio greater than 0.4.
References
1. Buckley SA, Reeves B, Burdon M, et al. Acute angle-closure glaucoma: relative failure of YAG iridotomy in affected eyes and factors influencing outcome. Br J Ophthalmol 1994; 78: 529-533.
2. Fleck BW, Wright E, Fairley EA. A randomized prospective comparison of operative peripheral iridectomy and Nd:YAG iridotomy treatment of acute angle-closure glaucoma: 3 year visual-acuity and intraocular-pressure-control outcome. Br J Ophthalmol 1997; 81: 884-888.
3. Aung T, Ang LP, Chan SP, Chew PTK. Acute primary angle closure: long-term intraocular-pressure outcome in Asian eyes. Am J Ophthalmol 2001; 131: 7-12.
4. Alsagoff Z, Aung T, Ang PK, Chew PTK. Long-term clinical course of primary angleclosure glaucoma in an Asian population. Ophthalmology 2000; 107: 2300-2304.
5. Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma: The Beaver Dam Eye Study. Ophthalmology 1992; 99: 1499-1504.
6. Foster PJ, Johnson GJ. Glaucoma in China: how big is the problem? Br J Ophthalmol 2001; 85: 1227-1282.
7. Foster PJ, Baasanhu J, Alsbirk PH, et al. Glaucoma in Mongolia: A populationbased survey in Hovsgol province, Northern Mongolia. Arch Ophthalmol 1996; 114: 1235-1241.
8. Foster PJ, Oen FTS, Machin D, et al. The prevalence of glaucoma in Chinese residents of Singapore: a cross-sectional population survey of the Tanjong Pagar district. Arch Ophthalmol 2000; 118:1105-1111.
9. Seah SKL, Foster PJ, Chew PTK, et al. Incidence of acute primary angle-closure glaucoma in Singapore: an island-wide survey. Arch Ophthalmol 1997; 115: 1436- 1440.
10. Lai JSM, Liu DTL,Tham CCY, et al. Epidemiology of acute primary angle-closure glaucoma in the Hong Kong Chinese population: prospective study. Hong Kong Med J 2001; 7: 118-123.
11. Congdon NG, Youlin Q, Quigley H, et al. Biometry and primary angle-closure glaucoma among Chinese, white, and black populations. Ophthalmology 1997; 104: 1489-1495.
12. George R, Paul PG, Baskaran M, et al. Ocular biometry in occludable angles and angle-closure glaucoma: a population based survey. Br J Ophthalmol 2003; 87: 399-402.
13. Wojciechowski R, Congdon N, Anninger W, et al. Age, gender, biometry, refractive error, and the anterior chamber angle among Alaskan Eskimos. Ophthalmology 2003; 110: 365-375.
14. Playfair TJ, Watson PG. Management of acute primary angle-closure glaucoma: a long-term follow-up of the results of peripheral iridectomy used as an initial procedure. Br J Ophthalmol 1979; 63: 17-22.
15. Saunders DC. Acute closed-angle glaucoma and Nd-YAG laser iridotomy. Br J Ophthalmol 1990; 74: 523-525.
16. Choong YF, Irfan S, Menage MJ. Acute angle-closure glaucoma: an evaluation of a protocol for acute treatment. Eye 1999; 13: 613-616.
17. Hsiao CH, Hsu CT, Shen SC, et al. Midterm follow-up of Nd:YAG laser iridotomy in Asian eyes. Ophthalmic Surg Lasers Imaging 2003; 34: 291-298.
18. Nolan WP, Foster PJ, Devereux JG, et al. YAG laser iridotomy treatment for primary angle closure in East Asian eyes. Br J Ophthalmol 2000; 84: 1255-1259.
19. Rosman M, Aung T, Ang LPK, et al. Chronic angle closure with glaucomatous damage: long-term clinical course in a North American population and comparison with an Asian population. Ophthalmology 2002; 109: 2227-2231

