Fulminant Acute Postoperative Endophthalmitis caused by Pseudomonas stutzeri in a Healthy Elderly Male
George Michael N. Sosuan, MD1, Kevin Kenjee K. Dee, MD1, Jomel G. Lapides, MD1,
Ruben Lim Bon Siong, MD1,2
1Department of Ophthalmology and Visual Sciences, University of the Philippines Manila-Philippine General Hospital, Manila
2Eye Institute, St. Luke’s Medical Center, Quezon City
Correspondence: George Michael N. Sosuan, MD
Office Address: Department of Ophthalmology and Visual Sciences, Sentro Oftalmologico Jose Rizal, Philippine General Hospital, University of the Philippines Manila, Taft Avenue, Ermita, Manila
Office Phone Number: +63285548400 local 8502
Email Address: gmsosuan@yahoo.com
Disclosures: The authors have no financial relationships or conflicts of interest to declare.
Although endophthalmitis after cataract surgery is rare, it is still the most dreaded complication since its outcome can be devastating, resulting in permanent vision loss.1 Postoperative endophthalmitis may be classified as acute (occurring within 6 weeks) or chronic (occurring after 6 weeks). Cataract surgery, one of the most common eye surgeries being performed worldwide, is largely responsible for most cases of acute endophthalmitis with reported incidences ranging from 0.03 to 0.2%. The majority of acute postoperative endophthalmitis occur within 7 days.2 In developed countries, 60% to 80% of these cases are caused by coagulase-negative staphylococcus, which is considered part of the normal flora of the ocular surface.1 However, in developing countries, Gram-negative microorganisms are an emerging cause of ocular infection, and are the most common bacterial isolates causing keratitis in the Philippines, India, and Thailand.3 In India, Gram-negative microorganisms make up nearly 40% of culture-proven acute postoperative endophthalmitis cases.4,5
Pseudomonas aeruginosa is the most common isolated and reported species of Gram-negative microorganisms causing acute postoperative endophthalmitis. It has been reported in isolated cases as well as in cluster outbreaks.6,7 In a recent review of endophthalmitis cases seen at the Philippine General Hospital, Pseudomonas aeruginosa was the most common cause of Gram-negative acute postoperative endophthalmitis.8 Other Pseudomonas species, like Pseudomonas stutzeri, have not been reported locally. To the best knowledge of the authors, this is the first reported case of acute postoperative endophthalmitis following cataract surgery due to Pseudomonas stutzeri in a healthy elderly male.
CASE REPORT
A non-hypertensive, non-diabetic male in his late 60s consulted due to eye pain and blurred vision after an uncomplicated cataract surgery on his left eye. Five days prior to consult, he underwent extracapsular cataract extraction (ECCE) with posterior chamber intraocular lens implantation (PCIOL) on the left eye for a brunescent nuclear cataract at an ambulatory eye surgical center. On day 1 after surgery, he reported some improvement in vision with good compliance to his postoperative medications (prednisolone acetate 1% eye drops 6x/day, moxifloxacin 0.5% eye drops 6x/day, and sodium chloride 5% eye drops 6x/day). He had elevated intraocular pressure (IOP) on his operated eye and was advised to follow up after 2 days. In the interim, he noted the development of left eye pain, redness, discharge, and blurring of vision. He denied trauma and wetting of the eye after surgery. On his follow-up, hypopyon with severe conjunctival congestion and poor view of the fundus were observed. An assessment of acute postoperative endophthalmitis was made. He was then referred to our institution for further management.
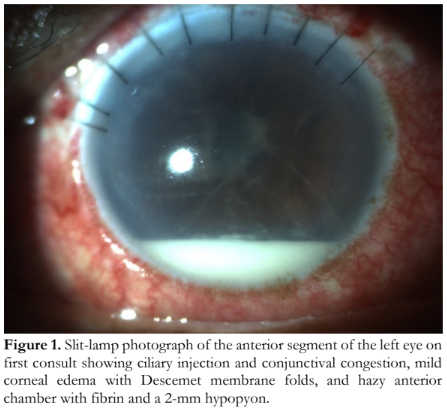
On examination, the visual acuity was counting fingers at 1 foot on the right eye and light perception on the left eye. A relative afferent pupillary defect (RAPD) was appreciated on the left eye. Slit-lamp examination of the left eye showed ciliary injection and conjunctival congestion, ten corneal slot sutures, mild corneal edema with Descemet membrane folds, hazy anterior chamber with fibrin and a 2-mm hypopyon, and a visible PCIOL (Figure 1). IOP was 10 mmHg with no leak on Seidel’s test. There was a poor view of the fundus. The right eye had a dense brunescent cataract. B-scan ultrasonography of the left eye showed dense vitreous cellularities and membranes consistent with endophthalmitis with no retinal detachment (Figure 2). Based on the collective findings, he was assessed to have acute postoperative infectious endophthalmitis following ECCE with PCIOL implantation.

Vitreous sampling was done for Gram staining, culture, and sensitivity tests, and intravitreal injections of vancomycin (1mg) and ceftazidime (2.25mg) were administered. Gram stain showed Gram-negative bacilli and numerous polymorphonuclear neutrophils. Topical levofloxacin 1.5% eye drops every hour, atropine sulfate 1% eye drops 3x/day, and intravenous ceftazidime were started. Pars plana vitrectomy, PCIOL explant, and intravitreal injection of antibiotics were subsequently done on the same day.
On postoperative day 1, vision improved slightly to hand movement with fair light projection with a persistent RAPD on the left eye. Slit-lamp examination showed slightly less ciliary injection and conjunctival congestion, mild corneal edema with epithelial defect and Descemet membrane folds, anterior chamber reaction of 2+ cells, and aphakia (Figure 3). IOP was 8 mmHg and view of the fundus remained poor. Hourly levofloxacin 1.5% eye drops, atropine sulfate 1% eye drops 3x/day, and intravenous ceftazidime were continued. Prednisolone acetate 1% eye drops 6x/day was started. The patient was then monitored closely.
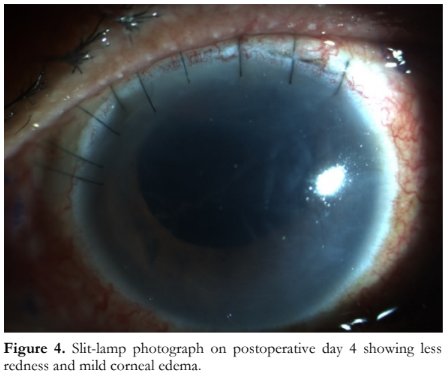
On postoperative day 4, vision was still at hand movement with fair light projection. Slit-lamp examination revealed less conjunctival redness, mild corneal edema, and less anterior chamber reaction of 1+ cells (Figure 4). The vitreous sample culture results revealed Pseudomonas stutzeri sensitive to ceftazidime and levofloxacin. Topical levofloxacin 1.5% eye drops was decreased to every 2 hours. Atropine sulfate 1% eye drops 3x/day and prednisolone acetate 1% eye drops 6x/day were continued. Intravenous ceftazidime was shifted to oral levofloxacin 750 mg once a day for another 7 days. The patient was sent home and instructed to follow up after 1 week.

On postoperative day 11, vision remained at hand movement with fair light projection. Slit-lamp examination showed clear cornea and quiet anterior chamber. Areas of retinal necrosis on the macula were noted. Topical levofloxacin 1.5% eye drop was decreased to 6x/day, atropine sulfate 1% eye drops was decreased to 1x/day, and prednisolone acetate 1% eye drops 6x/day was continued. The patient was asked to follow up after 2 weeks. On follow-up, the eye was hypotonic and fundus exam showed total retinal detachment. The plan for the left eye was control of inflammation and possible retinal detachment surgery. ECCE with PCIOL was contemplated for the right eye to provide functional vision to the patient.
DISCUSSION
This report describes a case of Pseudomonas stutzeri endophthalmitis in a non-hypertensive, non-diabetic, elderly male. Despite aggressive medical and surgical management, including topical, intravitreal, and systemic antibiotics, and emergency vitrectomy, vision loss was not prevented.
Pseudomonas stutzeri is an aerobic, motile, Gram- negative bacterium. It is ubiquitous and is found in soil, pond water, sewage, manure, and even in hospital environments. It rarely causes ocular infection, and isolation from ocular tissues has been reported only among immunocompromised and elderly patients.9 Pseudomonas stutzeri has been found to cause acute endophthalmitis after ECCE, trabeculectomy, and phacoemulsification. It can present as early as 2 days up to 2 years post- operatively and can occur in isolated cases and cluster outbreaks. Most patients with favorable visual outcomes presented and received appropriate treatment within 2 days postoperatively. Patients who presented after 2 days had severe clinical presentation and poor fundus visualization, and almost all of these patients had systemic comorbidities like diabetes mellitus.10 The diagnosis of acute postoperative endophthalmitis was made based on a high index of suspicion and confirmed by growth of the microorganism in culture. While polymerase chain reaction of the vitreous sample may yield faster confirmation, it is not widely available locally for ophthalmologic usage.11
The only identifiable risk factor for this patient was his age. The aging process affects both innate and adaptive immune responses resulting in increased susceptibility to infections.12 There were no wound leaks postoperatively and no posterior capsular rupture or vitreous loss intraoperatively. These are known conditions that may create a passageway for bacteria to gain access into the eye and cause infections. The authors postulate that the preoperative aseptic procedure might have been inadequate or a break in the aseptic technique might have occurred intraoperatively. Microbiological samples from the operating room environment (e.g. water, scrub sink, faucet, walls, floors, instrument trolleys, and microscope surfaces) and the devices used during the surgery should have ideally been obtained for analysis to determine cross-contamination.
Unfortunately, the patient presented 2 days after the surgery, thus precluding a favorable visual outcome despite aggressive medical and surgical management. Pseudomonas stutzeri is a motile bacterium with numerous virulence factors and is capable of spreading rapidly in an avascular environment like the cornea and vitreous. In addition, virulence factors such as the outer membrane protein also help these bacteria evade the immune system resulting to faster progression of infection.13
Acute postoperative endophthalmitis from Pseudomonas stutzeri is rare; if not recognized and treated promptly, this complication has devastating outcomes. It may present with a fulminant course regardless of the associated risks for infection. Prevention, early recognition, and timely management can prevent unfavorable visual outcomes.
REFERENCES
- Kresloff MS, Castellarin AA, Zarbin MA. Endophthalmitis. Surv Ophthalmol 1998;43: 193–224.
- Vaziri K, Schwartz SG, Kishor K, et al. Endophthalmitis: state of the art. Clin Ophthalmol 2015;9:95–108.
- Khor W-B, Prajna VN, Garg P, et al. The Asia Cornea Society Infectious Keratitis Study: a prospective multicenter study of infectious keratitis in Asia. Am J Ophthalmol 2018;195:161-170.
- Lalitha P, Sengupta S, Ravindran RD, et al. A literature review and update on the incidence and microbiology spectrum of postcataract surgery endophthalmitis over past two decades in India. Indian J Ophthalmol 2017;65:673-7.
- Gupta A, Gupta V, Gupta A, et al. Spectrum and clinical profile of post cataract surgery endophthalmitis in north India. Indian J Ophthalmol 2003;51:139-45.
- Zaluski S, Clayman HM, Karsenti G, et al. Pseudomonas aeruginosa endophthalmitis caused by contamination of the internal fluid pathways of a phacoemulsifier. J Cataract Refract Surg 1999;25:540–5.
- Hoffmann KK, Weber DJ, Gergen MF, et al. Pseudomonas aeruginosa-related postoperative endophthalmitis linked to a contaminated phacoemulsifier. Arch Ophthalmol 2002;120:90-3.
- Dimacali VG, Lim Bon Siong R. Infectious endophthalmitis at a Philippine tertiary hospital: a ten- year retrospective study. J Ophthalmic Inflamm Infect 2020;10:19.
- Holmes B. Identification and distribution of Pseudomonas stutzeri in clinical material. J Appl Bacteriol 1986; 60:401-11.
- Handa S, Singh SR, Sharma B, et al. Cluster outbreak of Pseudomonas stutzeri acute endophthalmitis following phacoemulsification: A report of 14 cases from North India. Indian J Ophthalmol 2022;70:2084-9.
- Al-Abri M, Al-Hinai A, Al-Farsi A, et al. Unusual case of endophthalmitis: case report and literature review. Oman J Ophthalmol 2020 Jan-Apr;13(1): 37-39.
- Castelo-Branco C, Soveral I. The immune system and aging: a review. Gynecol Endocrinol. 2014 Jan;30(1):16- 22.
- Azam MW, Khan AU. Updates on the pathogenicity status of Pseudomonas aeruginosa. Drug Discov Today 2019;14(1):350-59.

