Epithelial-Myoepithelial Carcinoma of the Lacrimal Gland – Case Report and Review of Literature
Mara Augustine S. Galang MD1, Gary John V. Mercado MD1,2, Armida L. Suller-Pansacola MD1,2,3, Jose M. Carnate Jr. MD4,5
1Department of Ophthalmology, Manila Doctors Hospital, Manila, Philippines
2Department of Ophthalmology and Visual Sciences, College of Medicine, University of the Philippines- Philippine General Hospital, Manila, Philippines
3Department of Ophthalmology, University of Santo Tomas Hospital, Manila, Philippines
4Department of Laboratory Medicine, Manila Doctors Hospital, Manila, Philippines
5Department of Pathology, College of Medicine, University of the Philippines-Philippine General Hospital, Manila, Philippines
Correspondence: Mara Augustine S. Galang, MD
Office Address: Department of Ophthalmology, Manila Doctors Hospital, 667 United Nations Avenue, Ermita, Manila, Philippines
Office Phone Number: +63 2 85580888 local 0557
Email Address: maraaugustinegalang@gmail.com
Disclosure: The authors report no financial conflict of interest.
Epithelial-myoepithelial carcinoma (EMC) is a low-grade neoplasm characterized by the pathognomonic biphasic morphology of inner cuboidal, densely eosinophilic luminal cells and outer layer of myoepithelial cells, which frequently have clear or vacuolated cytoplasm.1 This is supplemented by phenotypic immunohistochemical stain reactivity. EMC occurs primarily in the major salivary glands. In the Philippines, only one archival case arising from the parotid gland has been reported.2
Due to shared glandular structure and similar tumor characteristics, lacrimal gland carcinomas are classified using the World Health Organization (WHO) classification system for salivary gland tumors.1,3 To this date, only 10 cases of EMC of the lacrimal gland have been reported in the literature.4-13 Due to its infrequent occurrence, lacrimal gland EMC is not well understood in terms of its clinical behavior, presenting challenges in determining the most effective management strategies and predicting patient outcomes. In this report, we describe the first case of lacrimal gland EMC in a Filipino patient.
CASE PRESENTATION
A 31-year-old Filipino male seafarer had a 2-month history of an enlarging left superotemporal orbital mass. The growing mass was eventually accompanied by a decline in vision, diplopia, ptosis of the left upper eyelid, and inferonasal forward displacement of the left globe. There was no associated eye pain, headache, redness, or history of trauma. He consulted an ophthalmologist abroad and magnetic resonance imaging (MRI) was done which revealed a 3.6- x 3.0- x 2.2-centimeters (cm) circumscribed, lobulated, extraconal mass anterolaterally in the left orbit, with severe mass effect on the globe and lateral rectus muscle. There is focal invasion of the frontal process of zygomatic bone (Figure 1). The clinical impression then was a malignant lacrimal gland neoplasm, most likely adenoid cystic carcinoma. The patient was repatriated for further evaluation and management. He then consulted at our institution.
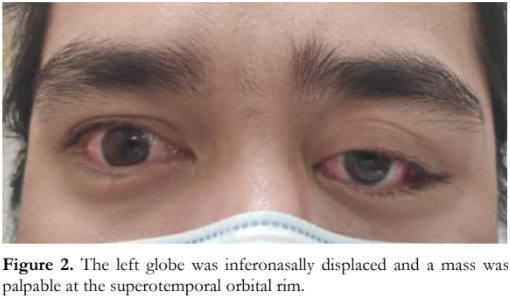
On examination, the visual acuity of the right eye was 20/20 and 20/40 on the left eye, with no relative afferent pupillary defect (RAPD). External eye and adnexa of the right eye were unremarkable. The left eye was displaced inferonasally and anteriorly, and a mass was palpable at the superotemporal orbital rim measuring 2.0 x 2.0 cm in its widest diameters (Figure 2). The mass was firm, non-movable, and non-tender to touch, with no associated skin ulceration, skin discoloration, or discharge. There were no external signs of infection or inflammation. Motility examination did not show deficits, but the patient reported diplopia on upgaze and left gaze. Slit lamp examination, Hertel’s exophthalmometry, tonometry, and fundus examination were not done due to the hospital’s coronavirus disease (COVID-19) protocols during the initial consult.
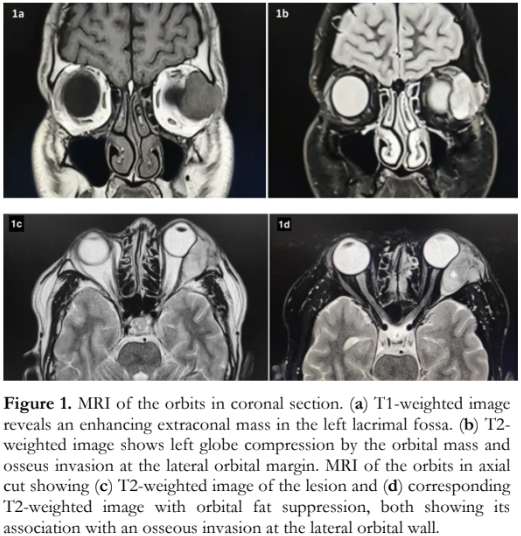
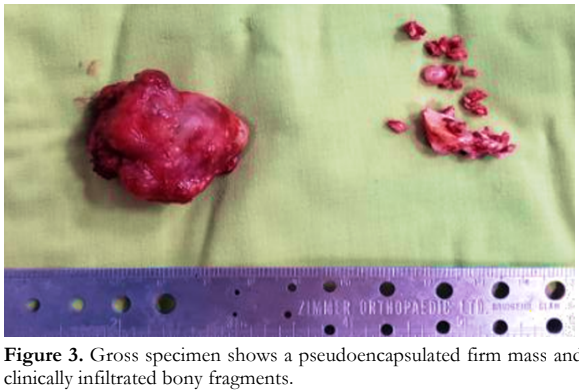
A modified Kronlein approach for lateral orbitotomy with en toto removal of the lesion along with the infiltrated lateral orbital bone were performed. Intraoperatively, a 3.8- x 3.0- x 3.0-cm multinodular, pseudoencapsulated, gray-brown, rubbery mass was obtained (Figure 3). The adjacent lateral bony margin was clinically infiltrated. Cut section of the mass showed tan-white solid surfaces with hemorrhage. The bone fragments consisted of varisized, tan-gray, irregular, hard, bony tissues with an aggregate dimension of 2.5 cm. Histopathologically, a diagnosis of EMC was rendered, 3 cm in greatest dimension with lymphovascular space and perineural invasion. Tumor cells were present in the surgical margins and bone fragments. Mitotic activity was rare with no areas of high-grade transformation.
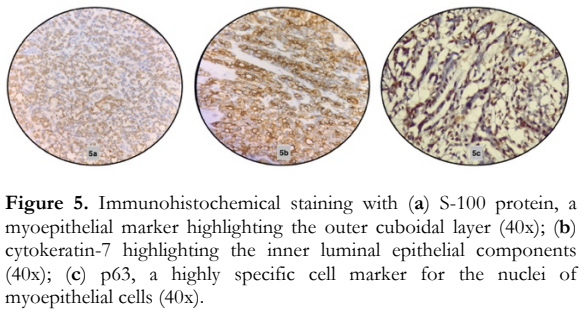
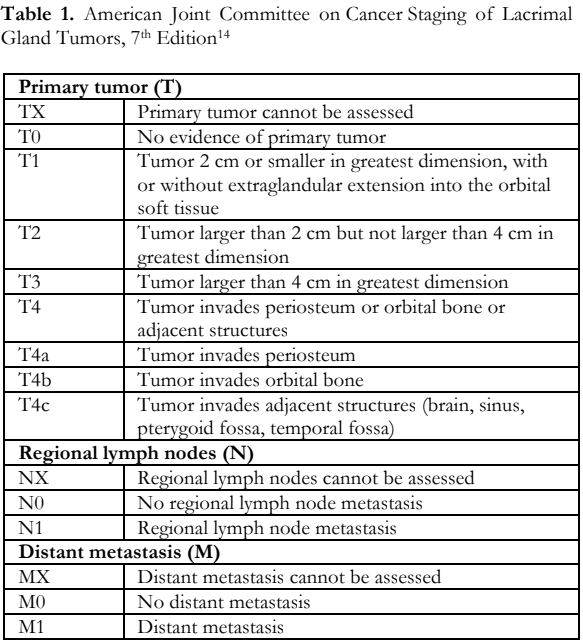
A histopathologic diagnosis of epithelial–myoepithelial lacrimal gland carcinoma was made based on the classic biphasic morphology of inner cuboidal luminal ductal epithelial cells and outer myoepithelial cells with abundant clear cytoplasm (Figure 4). Immunohistochemistry studies were done and confirmed the neoplasm. Reactivity for cytokeratin-7, S-100 and p63 were all positive in the appropriate cellular population in the tumor (Figure 5). Systemic surveillance using positron emission tomography (PET) and contrast computed tomography (CT) scan revealed no regional or distant metastasis. The American Joint Committee on Cancer (AJCC) guidelines stage (Table 1) for this case was T4bN0M0 based on the evidence of orbital bone invasion by the tumor (T4b) and the absence of regional lymph node metastasis (N0) and distant metastasis (M0).14
Adjuvant radiotherapy of the orbital area was performed for increased local control. Relatively radioresistant, positive margins need 66 Gray of intensity-modulated radiation therapy in 33 fractions which he completed at 14 weeks postoperatively. He had good wound healing but developed radiation- related keratopathy that was treated. At 8 months after completing adjuvant radiotherapy, the patient showed no evidence of tumor recurrence.

DISCUSSION
To our knowledge, this is the first case of an EMC of the lacrimal gland in a young Filipino male. Lacrimal gland tumors account for approximately 10% of all orbital masses, with epithelial tumors comprising 20-30% of all lacrimal gland tumors.15 The most frequent epithelial tumors are pleomorphic adenomas, adenoid cystic carcinomas, and adenocarcinomas.15 Lacrimal gland tumors share comparable morphology and clinical characteristics with salivary gland tumors; hence, the use of a similar classification system.
EMC is classified as a low-grade neoplasm, distinguished by its distinctive biphasic histology, and supported by the presence of specific immunostaining reactivity.3 EMC in the lacrimal gland is extremely rare with only 10 reported cases in the literature.4-13 In a study by Gore of 468 patients with EMC from various sites, only 1 case was found to occur in the lacrimal gland (0.2%).16 Most patients in the series were more than 50 years of age and there was female predominance with 1.6:1 ratio.16
There are 10 previously reported cases of lacrimal gland EMC.4,-13 Including our present case, the patients’ mean age at diagnosis is 58.7 years (range: 29-92 years), with male predilection at 60%.4-13 Five were reported from Asia, 4 from North America, and one each from Australia, and South America.4-13 Variable clinical presentations were noted including a palpable mass in the upper eyelid (100%), proptosis (81.8%), blurring of vision (81.8%), restricted extraocular motility (72.7%), inferior globe displacement (63.6%), diplopia (36.4%), ptosis of the upper eyelid (27.2%), eye redness (18.2%), chemosis (9.1%), eye pain (9.1%), eye discharge (9.1%), lagophthalmos (9.1%), tenderness on palpation (9.1%), headache (9.1%), RAPD (9.1%), optic disc edema (9.1%), and superior choroidal folds (9.1%).4-13 The mean duration of symptoms was 19.6 months (range: several days to 8 years ) before a diagnosis of EMC was made.4-13 Five out of the 11 cases (45.4%) arose from a preexisting pleomorphic adenoma, 5 were de novo lesions (45.4%) including our current case, while 1 (9.1%) arose from a lacrimal gland tumor of an unknown variant.4-13 Based on the AJCC Primary Tumor Staging (7th edition), 4 patients had T2 tumors (36.4%), 3 had T4b tumors (27.3%), 1 had a T3 tumor (9.1%), 1 had a T4a tumor (9.1%), 1 had a T1 tumor (9.1%), while the T status of 1 patient was unknown but appears T2-3 based on the description (9.1%).1,4-13 All patients were N0M0 on presentation.4-13 Eight out of the 11 patients (72.3%) underwent tumor excision via orbitotomy, 2 patients (18.2%) underwent orbital exenteration, and 1 had excision via frontoorbital craniotomy (9.1%) due to an area of bony erosion in the orbital roof and intracranial extension of the tumor without dural invasion.4-13 Adjuvant radiotherapy was done in 7 out of 11 patients (63.6%).4, 6, 10 Surveillance was done in all cases from 3 to 36 months (mean = 14.7 months).4-13
In the salivary gland, EMC is generally considered a low-grade malignancy with favorable survival rates.17 However, the existing literature does not provide a clear understanding of the outcome for EMC in the lacrimal gland. EMC can arise de novo, such as the case reported here or, less commonly, through the malignant transformation of a preexisting pleomorphic adenoma (EMC ex pleomorphic adenoma).4-13 Gonçalves et al. reported a similar case where they observed EMC of the lacrimal gland occurring 14 years after the complete excision of a pleomorphic adenoma.9 In another case by Venkatesulu et al., the patient had a third recurrence of lacrimal gland tumor after undergoing wide excision of pleomorphic adenoma two years and one year prior to presentation in their clinic.10 Three of the 5 patients with EMC ex pleomorphic adenoma had no previous lacrimal gland surgeries.4,6,12 On microscopic examination, EMC ex pleomorphic adenoma is composed of an infiltrative tumor and a benign component, characterized by bland ductal epithelial cells, myoepithelial cells, and stromal myxoid areas.6
Due to the limited number of reported cases of lacrimal gland EMC, it remains challenging to assess prognostic and predictive factors accurately. Most evidence is extrapolated from existing studies on salivary gland EMCs.4 In the context of salivary gland tumors, most cases exhibit an indolent course.17 Seethala et al. identified histopathologic features that confer a higher risk of recurrence in salivary and upper aerodigestive tract EMCs, namely positive surgical margins, angiolymphatic invasion, tumor necrosis, and myoepithelial anaplasia.17 Decreased survival was associated with age at diagnosis of over 80 years, black race, increasing AJCC TNM stage, and nonsurgical treatment.16 Adjuvant radiotherapy was found to decrease local recurrence and should be considered to those with positive margins, advanced primary disease, aggressive histopathological features, and regional lymph node metastasis.18 Although the benefits of adjuvant radiotherapy in lacrimal gland EMC is unknown, Skinner et al. found that recurrence was faster in patients with lacrimal apparatus malignancies who did not receive postoperative radiotherapy compared to those who received it.19 Therefore, it is prudent to consider radiotherapy in cases with positive margins or close margins, defined as tissue margins free of neoplastic cell less than 1 millimeter (mm), since it is often difficult to obtain clear or tumor-free margins within the orbit.4,10,19
The recurrence rate of EMC in the maxillofacial and sinonasal region are reported to range from 24 to 36.3%, while metastatic disease occurred in 5 to 11% of cases.17,18 There was no recurrence, regional/nodal metastasis, nor distant metastasis reported in the cases of lacrimal gland EMC at the last follow-up visit, including this case, even in the presence of positive surgical margins (72.7%), myoepithelial anaplasia (27.2%), tumor necrosis (18.2%) and lymphovascular invasion (9.1%), with our case the only one with this finding.4-13 Perineural invasion was found in 5 out of the 11 patients (45.4%), including this case, but such feature was found to have no significant impact in survival for EMC in salivary and upper aerodigestive tract.6,8,9,13,17
It is important to note, however, that most of the patients (6 out of 11 or 54.5%) with at least one of these histological features underwent adjuvant radiotherapy. 4,6-9 Three patients with positive surgical margins (27.2%) and one patient with perineural invasion but negative surgical margins (9.1%) did not undergo adjuvant radiotherapy.5,11,13 One out of the 11 patients (9.1%) underwent radiotherapy even with negative surgical margins.10
None of the 11 patients with lacrimal gland EMC were treated with neoadjuvant or adjuvant chemotherapy.4-13 There is limited data on the use of adjuvant chemotherapy in lacrimal gland carcinoma, but it can be added to radiotherapy following tumor excision or orbital exenteration if any of the following is present: tumor is larger than 2.5 cm, perineural invasion, positive margins, or orbital soft tissue or bone involvement.20 Neoadjuvant intra- arterial cytoreductive therapy followed by orbital exenteration, concurrent chemoradiation, and adjuvant radiotherapy has also been described in managing patients with lacrimal gland adenoid cystic carcinoma.21 In this series of 19 patients who underwent neoadjuvant chemotherapy, eight patients with an intact lacrimal artery had better survival (87.5% versus 14.3% at 15 years), disease-specific mortality, and recurrences than patients who received conventional treatment (surgical resection and adjuvant radiotherapy).21
The reported 5-, 10-, and 20-year survival rates for EMC arising from other sites are 73% to 94%, 60% to 90%, and 38%, respectively.16-18,22 One of the 10 patients with lacrimal gland EMC died at 15 months postoperatively with no clinical evidence of recurrent disease.7 The cause of death was unrelated to the lacrimal gland tumor, but no further information was provided by the authors.7
There are currently no standard guidelines in the management of lacrimal gland EMC. However, a multidisciplinary patient-centered approach is recommended.4 Malignant epithelial tumors of the lacrimal gland typically requires en bloc resection through lateral orbitotomy followed by postoperative radiotherapy to minimize the risk of local recurrence, which were done in this patient.4-7,9,11,13,20 The classic surgical treatment of malignant epithelial lacrimal gland tumors is orbital exenteration, which was done in the first reported case of lacrimal gland EMC by Ostrowski et al. in 1994.12 Orbital exenteration can also be considered in large, malignant tumors of the lacrimal gland measuring more than 2.5 cm in greatest dimension or those with orbital soft tissue infiltration or significant posterior orbital extension.20 These factors together with a suspicion of malignant transformation of a recurrent pleomorphic adenoma were taken into account prior to exenteration in the case reported by Venkatesulu et al.10 Similar to salivary gland EMC, complete surgical clearance is necessary, as incomplete resection increases the risk of recurrence, metastasis, and reduced survival.5,6,18
In summary, EMC, characterized by distinct histological and immunohistochemical features of epithelial and myoepithelial cells, is rarely found in the lacrimal gland with only 10 previously reported cases in the literature. Establishing an accurate diagnosis is crucial for initiating optimal treatment approaches. Lacrimal gland EMC seems to carry a fair prognosis, but long-term surveillance is needed to monitor for recurrence and metastasis. EMC of the lacrimal gland should be included in the differential diagnosis of infiltrative lesions in the lacrimal gland fossa.
REFERENCES
- Barnes L, Eveson JW, Reichart P, Sidransky D. Epithelial- myoepithelial carcinoma. In: Fonseca I, Soares J, eds. Pathology and Genetics of Head and Neck Tumours. Lyon, France: International Agency for Research in Cancer (IARC), 2003; 225.
- Carnate JM, Lapeña JFF. Epithelial-myoepithelial carcinoma of the salivary gland. Philipp J Otolaryngol Head Neck Surg. 2013 June;28(1):36-37.
- Grossniklaus H, Eberhart C, Kivela T. Rare neoplasms of the lacrimal gland. In: White VA, Chan ASY, Croxatto JO, Iacob CE, Salomao DR, Thaung C, eds. WHO Classification of Tumours of the Eye. 4th ed. Lyon, France: International Agency for Research in Cancer (IARC), 2018; 161.
- Van Rooij N, Newman AR, Vyas V, Sullivan TJ. A rare case of epithelial-myoepithelial carcinoma arising ex pleomorphic adenoma of the lacrimal gland: case report and review of the literature. Orbit. 2022 Dec;41(6):805-809.
- Chan WM, Liu DT, Lam LY, et al. Primary epithelial- myoepithelial carcinoma of the lacrimal gland. Arch Ophthalmol. 2004 Nov;122(11):1714-7.
- Avdagic E, Farber N, Katabi N, Shinder R. Carcinoma Ex Pleomorphic Adenoma of the Lacrimal Gland with Epithelial-Myoepithelial Carcinoma Histologic Type. Ophthalmic Plast Reconstr Surg. 2017 May/Jun;33(3S Suppl 1):S136-S138.
- Wiwatwongwana D, Berean KW, Dolman PJ, et al. Unusual carcinomas of the lacrimal gland: epithelial- myoepithelial carcinoma and myoepithelial carcinoma. Arch Ophthalmol. 2009 Aug;127(8):1054-6.
- Singh G, Sharma MC, Agarwal S, et al. Epithelial- myoepithelial carcinoma of the lacrimal gland: a rare case. Ann Diagn Pathol. 2012 Aug;16(4):292-7.
- Gonçalves AC, de Lima PP, Monteiro ML. Epithelial- Myoepithelial Carcinoma of the Lacrimal Gland 14 Years After En Bloc Resection of a Pleomorphic Lacrimal Gland Adenoma. Ophthalmic Plast Reconstr Surg. 2016 Mar- Apr;32(2):e42-4.
- Venkatesulu BP, Pathy S, Vallonthaiel AG, Chawla B. Epithelial-myoepithelial carcinoma of lacrimal gland from an ex pleomorphic adenoma. BMJ Case Rep. 2015 Jul 31;2015:bcr2015210795.
- Li E, Distefano A, Sinard J, Wong A, Pointdujour-Lim R. Epithelial-Myoepithelial Carcinoma Presenting as a Pseudo Veno-Lymphatic Malformation. Ophthalmic Plast Reconstr Surg. 2018 Sep/Oct;34(5):e157-e160.
- Ostrowski ML, Font RL, Halpern J, et al. Clear cell epithelial-myoepithelial carcinoma arising in pleomorphic adenoma of the lacrimal gland. Ophthalmology. 1994 May;101(5):925-30.
- Anjum S, Modaboyina S, Sen S, et al. Epithelial- myoepithelial carcinoma of the lacrimal gland: case report of youngest patient. Ophthalmic Plast Reconstr Surg. 2020 Nov/Dec;36(6):e141-e144.
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer, 2010.
- Alkatan HM, Al-Harkan DH, Al-Mutlaq M, et al. Epithelial lacrimal gland tumors: A comprehensive clinicopathologic review of 26 lesions with radiologic correlation. Saudi J Ophthalmol. 2014 Jan;28(1):49-57.
- Gore MR. Epithelial-myoepithelial carcinoma: a population-based survival analysis. BMC Ear Nose Throat Disord. 2018 Aug 16;18:15.
- Seethala RR, Barnes EL, Hunt JL. Epithelial-myoepithelial carcinoma: a review of the clinicopathologic spectrum and immunophenotypic characteristics in 61 tumors of the salivary glands and upper aerodigestive tract. Am J Surg Pathol. 2007 Jan;31(1):44-57.
- Wockner RS, Seethala RR, Emeto TI, et al. Epithelial- myoepithelial carcinoma of the maxillofacial and sinonasal region: a systematic review of presenting characteristics, treatment modalities, and associated outcomes. Int J Oral Maxillofac Surg. 2023 Jan;52(1):1-12.
- Skinner HD, Garden AS, Rosenthal DI, et al. Outcomes of malignant tumors of the lacrimal apparatus: the University of Texas MD Anderson Cancer Center experience. Cancer. 2011 Jun 15;117(12):2801-10.
- Woo KI, Yeom A, Esmaeli B. Management of Lacrimal Gland Carcinoma: Lessons From the Literature in the Past 40 Years. Ophthalmic Plast Reconstr Surg. 2016 Jan- Feb;32(1):1-10.
- Tse DT, Benedetto PW, Tse BC, Feuer WJ. Neoadjuvant Intra-Arterial Cytoreductive Chemotherapy for Lacrimal Gland Adenoid Cystic Carcinoma: A Long-Term Follow- up Study of a Trimodal Strategy. Am J Ophthalmol. 2022 Aug;240:239-251.
- Vázquez A, Patel TD, D’Aguillo CM, et al. Epithelial- Myoepithelial Carcinoma of the Salivary Glands: An Analysis of 246 Cases. Otolaryngol Head Neck Surg. 2015 Oct;153(4):569-74.

