Angle-supported intraocular-lens implantation for the correction of moderate to high myopia
Kevin Matthew Serafin B. Panggat, MD, Jesus Francisco, III, MD, Pik Sha Chan, MD, Harvey Uy, MD
METHODOLOGY
Thirteen eyes of 8 patients underwent angle-supported PIOL implantation at Asian Eye Institute (Makati, Philippines) from June 2009 to March 2010. Included into the study were patients who were at least 21 years old with moderate to high myopia (range, –7D to –16.5D). Excluded were eyes with an anterior-chamber
depth less than 3.2 millimeters (including corneal thickness), irregular or abnormal anterior-chamber anatomy, mesopic pupil diameter greater than 7 millimeters, nonqualifying preoperative endothelial-cell density according to age, preoperative astigmatism greater than 2.0D, stable manifest refraction of less than 1 year, chronic or recurrent anterior- or posterior-segment inflammation, existing cataract, retinal conditions or predisposition to retinal conditions, history of ocular trauma or intraocular surgery, and history of gl
aucoma and ocular hypertension. None of the eyes included previously received any eye medication. The patients gave their informed consent before initiation of any surgical procedure.
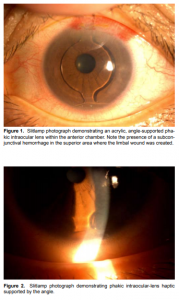
All underwent detailed history taking. Preoperative eye examination included assessment of uncorrected visual acuity (UCVA), best-corrected visual acuity (BCVA),manifest refraction, slitlamp biomicroscopy, applanation tonometry, dilated-fundus examination, and optical coherence tomography (OCT). Endothelial-cell counts were determined using specular microscopy (CellChek XL, Konan Medical Instruments, Hyogo, Japan). The PIOL power, model, and size needed to correct the refractive error were selected preoperatively. The PIOL power was determined using the online Acrysof Phakic Lens Calculator (www.phakiciolcalculator.com) which is based on the Van der Heijde equation. The PIOL size was based on the white-to-white diameter measured as the width of the cornea from the nasal limbus to the temporal limbus using an optical biometer (IOLMaster, Zeiss Meditec, Carlsbad, Germany). After aseptic preparation, the pupil was constricted using topical pilocarpine 2% (Isoptocarpine, Alcon Laboratories, Fort Worth, TX, USA) to protect the crystalline lens from potential contact with the PIOL. Proparacaine hydrochloride (Alcaine, Alcon Laboratories, Fort Worth TX) was instilled to anesthetize the eye. The white-towhite diameter was confirmed using intraoperative caliper. Side ports were created using a 15-degree blade. Ophthalmic viscoelastic device (OVD) consisting of sodium chondroitin sulfate 4% and sodium hyaluronate 1.65% (Provisc, Alcon Surgical, Fort Worth, TX, USA) was used to form and maintain the anterior chamber. Corneal tunnel incisions on the steep axis were created using a 3.4 millimeters keratome. The Acrysof Cachet PIOL, previously loaded into the IOL delivery-system cartridge, was injected midpupil using an IOL injector (Monarch, Alcon Surgical, Fort Worth, TX, USA). The distal haptics were positioned on the angle opposite the entry wound, the optic was delivered midpupil, and the proximal haptics was initially delivered just outside the incision and subsequently manually inserted into the anterior-chamber angle one haptic at a time using a dialing hook. All 4 haptics were positioned in the anteriorchamber angle. The remaining OVD was flushed out of the anterior chamber using a syringe filled with balanced saline solution. Vancomycin 5 mg was injected intracamerally, and lens position and wound integrity were inspected. No sutures were used. Postoperative medications consisted of gatifloxacin (Zymar, Allergan, Irvine, CA, USA) and prednisolone acetate (Pred Forte, Allergan, Irvine, CA, USA) eye drops applied 4 times daily for 4 weeks. All surgeries were performed by two surgeons (HSU and PCU). The eyes were examined 4 hours, 1, 7, 30 days after surgery, then quarterly. The following examinations were performed during the follow-up visits: UCVA, BCVA, slitlamp biomicroscopy, applanation tonometry, and dilated-fundus examination.

The lens position was assessed at each visit. UCVA, BCVA, manifest refraction,and specular microscopy were repeated on the firstmonth visit. Statistical analysis was performed using the SPSS 17.0 for Windows (SPSS, Chicago, IL, USA). Descriptive analysis was calculated for the variables and measured outcomes. Paired t-test was performed, with the probability level at <0.05 considered statistically significant, to compare baseline preoperative and postoperative values.
RESULTS
Mean patient age was 34.54 ± 10.5 years (range, 21 to 47 years) and mean postoperative follow-up period was 2.54 ± 1.39 months (range, 1 to 5 months). Mean preoperative spherical equivalent (SE) was –11.79 D (range, –18 to –6.75 D) and mean postoperative SE was –0.08 D (range, –1 to 0.88 D). The average change in SE was 11.71 D (p = 0.000) (Table 1). Mean preoperative UCVA was 0.016 (range, 0.01 to 0.05) and mean postoperative UCVA was 0.79 (range, 0.2 to 1.0). The average change in UCVA was +0.77 (p = 0.000) (Table 1). The preoperative UCVA was counting fingers in 11 eyes (85%) and 20/400 in 2 eyes (15%). The postoperative UCVA was 20/40 or better in 11 eyes (85%) and 20/20 was achieved in 7 eyes (53%). Two eyes had increased IOP 1 day postoperatively (30 and 36 mm Hg), which returned to normal the next day after treatment with timolol maleate eye drops applied twice daily. No other adverse events were observed.
DISCUSSION
Acrylic, angle-supported, PIOL implantation is a novel alternative to laser refractive surgery and refractive-lens exchange. PIOL produces acceptable refractive results for moderate and high myopes while preserving accommodative ability. The visual outcomes were excellent with 60.8% achieving an UCVA of 20/40 or better in a report by Alio5 and 99.4% in a report by Perez-Santoja.7 The efficacy and predictability in correcting myopia in this series were similar to those reported by other authors.4-7, 9-11 Results showed significant reduction of high myopia, stable refractive outcomes, and marked improvement in UCVA and BCVA after surgery. Fundamental requirements for achieving good visual outcomes are good surgical technique, small surgical incision, and accurate lens-power calculation. The availability of an online IOL calculator allows accurate and rapid determination of the appropriate lens power to achieve desired postsurgical refractive results. The effects of PIOL implantation on the corneal endothelium have been reported to be insignificant over as long as a 10-year follow-up period.3-12 In our study, the decrease in ECC was minimal. None of our subjects suffered a cell loss of more than 12% (mean ECC loss 8.4%). These results corroborated the findings of separate studies done by Alio,5 and Baikoff7 that suggested that the Alcon Acrysof Cachet, a hydrophobic acrylic, angle-supported PIOL, may be superior to other kinds of anterior-chamber PIOLs as it appeared to be better tolerated by the corneal endothelium. A recent publication revealed that the one-year ECC loss was –4.77% in a group of 139 eyes.13 Three-year Alcon data revealed annualized rate of central ECC loss at –0.41 to –0.91%.14 However, long-term examinations on large numbers of eyes are needed to establish long-term safety. Of special consideration is the accurate sizing of the PIOL. The use of inappropriate PIOL lengths may lead to PIOL instability and displacement. In a report by Coullet,8 3 eyes suffered from rapid and severe postoperative endothelial-cell loss that required PIOL removal and Descemet’s-membrane repair. The main risk factor identified was the implantation of oversized PIOLs that caused excessive vaulting into the anterior chamber. However, aging and accommodation may affect accurate PIOL sizing because aging changes can modify anteriorchamber internal diameters.5 Modern anterior-chamberbiometry methods may help improve accuracy of PIOLsize determination. In this case series, the incidence of increased IOP that required topical IOP lowering drugs (15%) was higher than that noted in another study (7.2%). This transient increase in IOP occurring right after implantation was recognized to be related to retained OVD.8 Since these were initial cases, OVD may not have been removed completely but adjustments in washout techniques in subsequent patients led to the elimination of IOP rise. There were no cases of elevated IOP after the first-day postoperative visit. These findings highlight the need for careful OVD removal during surgery and close monitoring in the immediate postoperative period. Other adverse events that occurred in other reports, such as IOL dislocation, corneal haze, secondary glaucoma, iris atrophy, cataract development, pupil ovalization, retinal detachment, and endophthalmitis,3, 7-11 were not seen in this study. A comprehensive preoperative workup, good surgical technique, and proper loading of PIOL avoided such adverse events. Two cases were reported in another study wherein the PIOLs were implanted upside down resulting in iatrogenic cataracts.8 The ensuing addition of side-up indicators in the PIOL structure eliminated the risk of improper loading of the PIOL. In conclusion, acrylic, angle-supported, PIOL implantation produces excellent, predictable, and stable refractive outcomes in moderate to high myopic eyes after a mean follow-up of 2.5 months. PIOL implantation is a safe technique that can be easily learned and requires few additional instruments for incorporation into a practice.
Dirani M, Chan YH, Hornbeak DM, et al. Prevalence of refractive error in Singaporean Chinese children: the strabismus, amblyopia, and refractrive error in young Singaporean Children (STARS) study. Invest Ophthalmol Vis Sci 2010; 51: 1348-1355.
Melki SA, Azar DT. LASIK complications: etiology, management, and prevention. Surv Ophthalmol 2001; 46: 95-116.
Huang D, Schallhorn SC, Sugar A., et al. Phakic intraocular-lens implantation for the correction of myopia: a report by the American Academy of Ophthalmology. Ophthalmology 2009; 116: 2244-2258.
Allemann N, Chamon W, Tanaka W. et al. Myopic angle-supported intraocular lenses: two-year follow-up. Ophthalmology 2000; 107: 1549-1554.
Alio JL, de la Hoz F, Perez-Santonja JJ, et al. Phakic anterior-chamber lenses for the correction of myopia: a 7-year cumulative analysis of complications in 263 cases. Ophthalmology 1999; 106: 458-466.
Coullet J, Mahieu L, Malecaze F, et al. Severe endothelial-cell loss following uneventful angle-supported phakic-intraocular-lens implantation for high myopia. J Cataract Refract Surg 2007; 33: 1477-1481.
Pérez-Santonja JJ, Alió JL, Jimenez-Alfaro I, et al. Surgical correction of severe myopia with an angle-supported phakic intraocular lens. J Cataract Refract Surg 2000; 26: 1288-1302.
Arne JL, Lesueur LC. Phakic posterior-chamber lenses for high myopia: functional and anatomical outcomes. J Cataract Refract Surg 2000; 26: 369-74.
Baikoff G, Arne JL, Bokobza Y, et al. Angle-fixated anterior-chamber phakic intraocular lens for myopia of -7 to -19 diopters. J Refract Surg 1998; 14: 282- 293.
Mimouni F, Colin J, Koffi V, Bonnet P. Damage to the corneal endothelium from anterior-chamber intraocular lenses in phakic myopic eyes. Refract Corneal Surg 1991; 7: 277-281.
Benedetti S, Casamenti VJ, Marcaccio L, et al. Long-term endothelial changes in phakic eyes after Artisan intraocular-lens implantation to correct myopia: five-year study. J Cataract Refract Surg 2007; 33: 784-790.
Saxena R, Boekhoorn SS, Mulder PG, et al. Long-term follow-up of endothelial cell change after Artisan phakic intraocular lens implantation. Ophthalmology 2008; 115: 608-613.
Kohnen T, Knorz MC, Cochener B, et al. Acyrsof phakic angle-supported intraocular lens for the correction of moderate to high myopia: one-year results of a multicenter European study. Ophthalmology 2009; 116: 1314-1321.
Clinical Integrated Summary of Clinical Studies of the Acrysof CACHET Angle- Supported IOL (L Series). DC# 0000822366. Alcon Company Data on file.

