A Simplified Xeroscope for the Noninvasive Measurement of Tear Break-up Time
Darien B. Gaw, MD, Bernard Gil O. Tinio, MD
A stable and continuous tear film is essential for achieving an optically smooth corneal surface and maintaining optimal visual acuity. Prolonged gaze without blinking results in tear film instability, which is associated with decreased functional visual acuity in patients with dry eyes.1
The stability of tear film depends on many factors, such as intact blink reflex mechanisms, presence of healthy lacrimal glandular tissue, and structurally intact tear film. All three layers of the tear film, the mucin, aqueous, and lipid layers, significantly contribute to tear stability. Qualitative and quantitative disorders of any of these layers can affect the tear film stability immensely.2
There are many well-established techniques and tests for diagnosing dry eye. One of the major diagnostic tests for identifying global features of dry eye is the assessment of tear film surface quality and the measurement of tear film break-up time (TBUT). This test involves observing the cornea after a complete blink, and measuring the interval from that blink to the first evidence of a discontinuity in the tear film. This is most easily visualized by instilling 0.1% sodium fluorescein into the conjunctival cul-de-sac. The patient is then asked to blink several times to assure mixing of the fluorescein with the tear film. The clinician examines the precorneal tear film with a slit-lamp using a cobalt blue filter. The TBUT is the time, in seconds, from that blink to the appearance of the first dry spot. Values below 10 seconds are generally considered abnormal.2
TBUT assessment is still favored by many eye clinicians today as it is repeatable and minimally invasive. However, the instillation of fluorescein dye can destabilize the tear film.3 As pointed out by Mengher et al, the addition of any substance to the tears will decrease their stability and may produce biased and unreliable estimates of tear film break-up times.4
The measurement of TBUT in the absence of fluorescein can overcome this problem and give a more accurate assessment of tear stability.5 Hence, significant efforts have been made toward developing methods of noninvasive assessment of tear film surface quality, from which estimates of tear film break-up time could be derived.
In 1985, Mengher et al developed a simple, non-invasive technique of evaluating the stability of precorneal tear film. It involved specular reflection of a grid pattern from the tear film surface in which breaks appear as random discontinuities in the grid image. This non-invasive technique provided an alternative approach in diagnosing dry eye disorders, as well as a means of evaluating the efficacy of artificial tear solutions.6
Madden et al compared HIR-CAL grid-modified keratometer and the Mengher-Tonge xeroscope in 1994. It sought to establish the repeatability and inter-observer variability of the two devices.7 Ounnoughene and Nichols made separate evaluations on Tearscope Plus™ (Keeler, USA) which basically adopted the same principle of grid specular reflection with an additional still photograph and video capability with improved accuracy in mind.8,9 Recently, Jones and Nischal conducted a study on noninvasive tear break-up time (NIBUT) in children using Keeler Tearscope™ in which they found that NIBUT was greater in children in comparison to adults.10
Research into noninvasive methods resulted in the development of techniques and devices for the assessment of precorneal tear film break-up time. However, use of noninvasive methods did not find widespread acceptance due to the high cost of commercially-available xeroscopes and tearscopes in our locality which cost around one hundred to five hundred thousand pesos depending on the model and device specifications.
In 2007, using the same basic concept behind grid xeroscopes and tearscopes, a study by Herrera and Lim Bon Siong proposed a prototype xeroscope made from readily available materials as a simple, noninvasive, and inexpensive alternative to measure NIBUT. It used a halogen flashlight which projected simulated placido rings on the precorneal surface.11 However, the design offered limited corneal coverage and might have also caused reflex tearing as bright light was shone on the subjects’ eyes, thereby causing possible bias and inaccuracy. It was on this account that our study was directed towards proposing a new ingenious prototype design to counter these limitations, yet still use cheap and locally available materials which can be bought at a local electronic hardware or Do-It-Yourself (DIY) shops.
METHODOLOGY
This study was conducted in accordance with the international standards of Good Clinical Practice, applicable government regulations, and institutional research policies and procedures
Prototype Xeroscope
A prototype xeroscope was made from an ordinary round plastic lid. Fixed in its inner surface was a fluorescent green sticker paper with printed concentric grid pattern. A central rectangular cut-out measuring 2 cm x 1 cm was made into the base frame serving as the viewfinder for the device through which the precorneal grid reflection was observed (Figure 1).A 30-LED blue light strip was fixed in the inner rim of the lid using a double-sided adhesive tape. The light source was connected to a multi-voltage AC-DC power adapter set at 6-volts and 350mA (2 watts). A black light enclosure fashioned from a piece of cardboard was fitted and fixed over the lid rim to minimize light scatter (Figure 2).
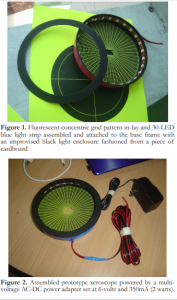
Figure 1. Fluorescent concentric grid pattern in-lay and 30-LED blue light strip assembled and attached to the base frame with an improvised black light enclosure fashioned from a piece of cardboard.
Figure 2. Assembled prototype xeroscope powered by a multi-voltage AC-DC power adapter set at 6-volts and 350mA (2 watts).
Participants
Fifty (50) patients (100 eyes), aged 21 to 65 years old, with no apparent ocular surface disorders and eyelid abnormalities were recruited from the ophthalmology outpatient department of Makati Medical Center-Hospital Service Program and enrolled consecutively into the study. A complete ocular history and basic eye evaluation that included visual acuity testing and adnexal slit-lamp biomicroscopy examination were performed to all potential participants to ascertain eligibility. Excluded were patients currently using any ophthalmic medication or contact lenses, those who underwent any ocular surgery, and those with a history of penetrating eye trauma. The study was approved by the Institutional Review Board of Makati Medical Center and informed consent was obtained from all participants prior to the conduct of the study.
Noninvasive Tear Break-up Time (NIBUT)
Each eligible participant was seated in front of a Haag-Streit-type slit-lamp biomicroscope with an illuminating arm (Appasaamy A-010) set at 10x magnification. He was instructed to fixate at the center of the prototype xeroscope at all times, which was positioned in front of the eye and attached to the illumination arm of the slit-lamp via a magnet (Figure 3). He was asked to make a complete blink and keep the eyes open while NIBUT was measured. The precorneal grid reflection was observed through the oculars of the slit-lamp by the examiner. Ambient light sources other than that of the device were switched off to enhance the precorneal specular reflection.
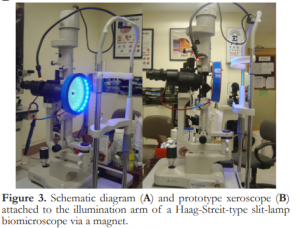
Figure 3. Schematic diagram (A) and prototype xeroscope (B) attached to the illumination arm of a Haag-Streit-type slit-lamp biomicroscope via a magnet.
The time interval between a complete blink and the appearance of the first randomly distributed progressive distortion on the precorneal concentric grid reflection (Figure 4) was observed using a designated digital stopwatch. This interval was labelled as the noninvasive tear break-up time (NIBUT). The test was repeated three times on both eyes. Mean NIBUTs from both eyes were recorded.

Figure 4. Projected corneal concentric grid pattern seen during NIBUT measurement through a slit-lamp biomicroscope: with intact tear film (A) and with distorted grid pattern signifying presence of dry spots (B).
A 10-minute rest period was given to all participants before proceeding to TBUT measurement. This was to ensure that no residual tearing was carried over to the next procedure.
Invasive Tear Break-up Time (TBUT)
Sterile single-use 1 mg fluorescein sodium ophthalmic strips (Fluostrip™) were used for TBUT assessment. The fluorescein-impregnated tip of the Fluostrip™ was moistened with two (2) drops of sterile balance salt solution (Alcon™ BSS®). The tip was tapped lightly across the bulbar conjunctiva just above the superior limbus. Patient was asked to blink a few times to spread the dye evenly across the eye.
Using the same slit-lamp with its built-in cobalt blue filter on, subjects were asked to make a complete blink and told to keep their eyes open as TBUT was measured. The interval between the complete blink and the appearance of the first randomly distributed progressive dark areas or dry spots in the precorneal tear film (Figure 5) was measured in seconds using the same digital stopwatch and was designated as the fluorescein tear break-up time (TBUT). The test was repeated three times on both eyes and the mean TBUTs were recorded.
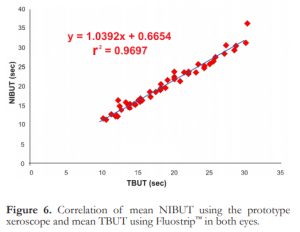
Figure 5. Fluorescein TBUT assessment: with intact tear film (A) and with dry spots (B).
All the tests were done by a single examiner, using the same slit-lamp biomicroscope under standard room conditions.
Data Analysis
The mean NIBUT and TBUT from each eye were compared and their correlation determined by linear regression.
RESULTS
Fifty (50) patients (100 eyes) composed of sixteen (16) males and thirty-four (34) females were enrolled into the study. Mean age was 48.52 years (range: 21 – 62 years). All subjects had no ocular surface disorders, eyelid abnormalities, nor were on any maintenance ophthalmic medication. No withdrawals were encountered during the course of the study.
The combined mean NIBUT of both eyes was 20.10 seconds, ranging from 11.56 to 36.17 seconds. The combined mean TBUT was 18.70 seconds, ranging from 10.20 to 30.40 seconds. There was a strong positive linear correlation between NIBUT and TBUT of both eyes (Figure 6) and the mean difference was 1.40 second with a computed R-squared value of 0.9697. Using the computed y intercept in the regression analysis, γ=1.0392x + 0.6654, the predicted normal NIBUT for the prototype device was 11.06 seconds basing on the generally accepted normal TBUT value of 10 seconds.
DISCUSSION
Maintaining a healthy and comfortable ocular surface requires stability and constant renewal of the preocular tear film. Eye disorders that disturb tear volume, composition, and hydrodynamic factors can threaten this stability. An unstable tear film is the hallmark of various dry-eye states. Patients with tear-film instability complain of annoying eye irritations and may experience constant and disabling eye irritation, and develop ocular-surface epitheliopathy.
Measurement of tear-breakup time (TBUT) using fluorescein dye is the most widely used clinical assessment of tear-film stability. Values below 10 seconds are generally considered abnormal. Measuring TBUT using fluorescein is inexpensive, readily available, and easy to perform. On the other hand, instillation of the dye may alter the physiologic composition and volume of the tear film, as well as induce reflex tearing, affecting the real or physiologic TBUT. Thus, it is classified as an invasive type of measurement.
Noninvasive techniques and devices have been developed to measure TBUT.12-13 The Keeler Tearscope Plus and Oculus Keratograph14 are examples of these devices that can estimate tear-film quality, quantity, and stability. All are designed to avoid any disruption of the tear film during examination and to give a single view of the whole cornea. Goto and colleagues evaluated the videokeratography machine, which was used for corneal topography and for the measurement of TBUT.3 Results were consistent with those of the standard fluorescein TBUT. All these sophisticated machines use placido rings or grid lines projected onto the corneal surface to measure tear-film stability. These machines, however, are quite expensive and are neither practical nor readily available to most local ophthalmologists.
The prototype xeroscope used in our study was made from simple, cheap, locally-sourced materials at a cost of less than Php1,000. The design was simple and straightforward, meant towards creating a good visible precorneal grid reflection using minimal ambient light at a fraction of the cost of today’s xeroscopes and tearscopes.
Our data showed a strong positive linear correlation between NIBUT measurements obtained using the prototype device and the standard fluorescein TBUT measurements, with a mean difference of only a second between the two test procedures. This indicated that the prototype device could be used as an alternative to current standards for measuring stability of the tear film noninvasively. The use of fine luminescent concentric grid pattern further enhanced detection of dry spots with minimal ambient light; thereby, minimizing reflex tearing.
In conclusion, the prototype xeroscope provided a good alternative for measuring tear-film stability without disturbing normal tear physiology and dynamics. However, it was recommended that the prototype device be used with slitlamp biomicroscopes with video recording capability and time in-lay to further improve the accuracy of NIBUT measurements.
REFERENCES
1. Djalilian A, Hamrah P, Pflugfelder S. Dry Eye. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea, 2nd ed. Philadelphia: Mosby-Year Book, Inc. 1997. v1, chap. 42.
2. Dry Eye Tests and Eye Exam. The Eye Digest. University of Illinois Eye & Ear Infirmary, Chicago, IL. Jun 2007. http://www.agingeye.net/dryeyesdryeeyeseyeexam.php.
3. Goto T, Zheng X, Klyce SD, et al. A new method for tear-film-stability analysis using videokeratography. Am J Ophthalmol 2003;135:607-612.
4. Mengher LS, Pandher KS, Bron AJ. Noninvasive tear-film breakup time: sensitivity and specificity. Acta Ophthalmol 1986; 64:441-444.
5. Kojima T, Ishida R, Dogru M, et al. A new noninvasive tear-stability-analysis system for the assessment of dry eyes. Invest Ophthalmol Vis Sci 2004;45:1369-1374.
6. Mengher LS, Bron AJ, Tonge SR, Gilbert DJ. A noninvasive instrument for clinical assessment of the precorneal tear film stability. Curr Eye Res 1985;4:1-7.
7. Madden RK, Paugh JR, Wang C. Comparative study of two noninvasive tear film techniques. Curr Eye Res 1994;13:263-269.
8. Ounnoughene Y, Benhatchi N, Agboke K, et al. The video tearscope: A new method for evaluating lacrimal film in vivo. J Fr Ophthalmol 2006;29:476-484.
9. Nichols JJ, Nichols K, Puent B, et al. Evaluation of tear film interference patterns and measures of tear break-up time. Optom Vis Sci 2002;79:363-369.
10. Jones SM, Nischal KK. The noninvasive tear film break-up time in normal children. Br J Ophthalmol 2013;97:1129-1133.
11. Herrera JED, Lim Bon Siong R. A prototype xeroscope for the noninvasive measurement of tear-breakup time. Phillip J Ophthalmol 2007;32:70-75.
12. Szczesna DH, Alonso-Caneiro D, Iskander RD, et al. Predicting dry eye using noninvasive techniques of tear film surface assessment. Invest Ophthalmol Vis Sci 2011;52:751-756.
13. Gumus K, Crockett CH, Rao K, et al. Noninvasive assessment of tear stability with the Tear Stability Analysis System in tear dysfunction patients. Invest Ophthalmol Vis Sci 2011;52:456-461.
14. Best N, Drury L, Wolffsohn JS. Clinical evaluation of the Oculus Keratograph. Contact Lens Anterior Eye 2012;35:171-174.

