Transcripts of the 2021 Dr. Romeo B. Espiritu Memorial Lecture: The Evolving Management of Diabetic Retinopathy
Harvey S. Uy, MD1,2
1Peregrine Eye and Laser Institute, Makati City
2College of Medicine, University of the Philippines, Manila
Correspondence: Harvey S. Uy, MD
Clinic Address: Peregrine Eye and Laser Institute, Morning Star Centre, 347 Sen. G. Puyat Ave, Bel-Air, Makati Clinic Phone Number: +632-8890-0115
Email Address: harveyuy@yahoo.com
Disclaimer: The author reports no financial disclosures.
In Greek mythology, Orion was a son of Poseidon and a giant-sized hunter. Orion visited the Greek island of Chios and was invited to a party by Oenopion, the king of Chios. King Oenopion is credited as the inventor of winemaking which is why wine lovers like Dr. Pearl Villalon are called oenophiles. According to legend, Orion got drunk with the wine of Oenopion at that party and attempted to rape Oenopion’s daughter. In anger, Oenopion gouged out the eyes of Orion and rendered him blind. This is the first recorded enucleation procedure in history. Not being able to see, Orion then stumbled around. Hephestus, the Greek god of the Forge, took pity on him and asked his servant to step on the shoulders of Orion and guide Orion to the east until he found the sun god, Helios, who restored his eyesight by shining a light on his face—the first recorded case of laser treatment. This story was the origin of the phrase, “standing on the shoulders of giants”. In this presentation, I’m privileged to be standing on the shoulders of giants, our mentors, and to share with you a vision on the past and future of diabetic retinopathy (DR) management.
We will be suffering the effects of the COVID-19 pandemic for a long time but eventually the pandemic will end. There is another more insidious, and perhaps longer-lasting, pandemic going on. This is the diabetes pandemic that is continually spreading around the world. The number of diabetics in Southeast Asia alone is expected to double in the next 30 years. Of the 93 million people worldwide with DR, one third are at risk for losing their eyesight.1,2,3 Among the different retinal diseases, DR generates the greatest interest. Over the next half hour, we will be reviewing the evolution and future trends in DR management.
DR arises from a complex series of biochemical events induced by hyperglycemia resulting in the upregulation of harmful cytokines, such as vascular endothelial growth factors (VEGF), resulting in the dysregulation of normal vascular function.4 Over time, these vascular changes lead to progressive worsening of the disease and eventual visual loss.
Narcissus was a handsome young man who fell in love with the reflection of himself in a pond. He became so obsessed with his self-image that he spent all of his time looking at his reflection in the pond until he died from starvation. Looking at images is not all bad and imaging has become vital for the diagnosis and management of DR.
The earliest steps in DR management were in the field of imaging which ushered in a golden age in ophthalmology. Von Helmholtz’s invention of the direct ophthalmoscope in 1850 allowed visualization of retinal diseases. This invention was revolutionary and likely one of the reasons why our Philippine national hero, Dr. Jose Rizal, traveled to Germany to study ophthalmology. A decade later, in 1861, Maxwell developed fundus photography that allowed photographic capture of retinal images. A century later, in 1945, Schepens popularized the indirect ophthalmoscope which allowed wide-angle viewing of the fundus. Fluorescein angiography was invented by Novotny and Alvis in 1961. Novotny’s paper on fluorescein angiography was rejected by all the ophthalmology journals at that time and was finally published in a cardiology journal, “Circulation”. Recently, non-contact ultrawide field fluorescein angiography and photography have allowed visualization of nearly the entire retina. All of these imaging advances have allowed eye doctors over time to visualize the changes of the diabetic retina and develop theories on its pathophysiology as well as its treatment. Imaging has also led to grading systems for diabetic macular edema and DR severity which allow us to monitor disease progress and responses to treatment.
Imaging and treatment now go hand in hand. The original fundus cameras only imaged the posterior pole. Standard wide field cameras capture 75 degrees of the fundus while ultrawide field cameras now allow imaging up to 200 degrees. We should be proud that one of our Filipino ophthalmologists, Dr. Paolo Silva, is now a leader in this field.5 The introduction of optical coherence tomography (OCT) and OCT-angiography has been revolutionary, providing us with a non-invasive, well accepted, and quantitative method for imaging of the retina. OCT has allowed us to monitor treatment response as well as identify biomarkers that may be used in prognosticating disease treatment and final visual outcome. OCT-angiography is a recent and amazing non-invasive technique that can identify retinal vasculature by analyzing changes in consecutive OCT scans. OCT-angiography allows identification of vascular structures without contrast dyes. This imaging modality is useful in DR where ischemia and new vessel growth are markers of disease progression.
The next advances in DR management were in the field of laser technology. In Greek mythology, Phaeton was an ambitious human son of Helios who yearned to drive the fiery carriage of his dad. Unfortunately, he lost control and rapidly burned up the earth with his carriage—the first report of human-induced climate change. Gaia, or Mother Earth, sought the help of Zeus, who used a bolt of lightning to strike down Phaeton, thus saving the Earth. Mankind has always sought to harness the power of light energy and this was realized with the first effective treatments for DR using lasers.
The invention of lasers ushered in a new age of treatment for retinal diseases and the beginnings of evidence-based ophthalmology. The landmark DR study involved multiple eye centers working together on a single research question, using a standardized definition of diseases, a standardized treatment, and standardized staging. The main finding of this study was that laser treatment reduces the risk of blindness by half among eyes with high-risk DR.6,7 Panretinal photocoagulation (PRP) is still the standard of care for proliferative disease 40 years later.
The next landmark study was the Early Treatment for Diabetic Retinopathy Study (ETDRS) that introduced a standard set of definitions for clinically-significant macular edema.8 The ETDRS determined that focal or grid laser treatment reduced the rate of visual loss in eyes with diabetic macular edema (DME). This became the gold standard treatment for many years. Laser treatment, while effective for reducing the rate of visual loss, does not come without a price. Patients who undergo laser treatment are at risk for visual field contraction, poorer nighttime vision, and vision loss resulting from laser scars extending into the fovea.9 Moreover, some patients continue to have visual decline due to incomplete drying up of the macula after laser treatment.
The first ophthalmic lasers applied energy over a long pulse duration of more than 100 milliseconds which often caused discomfort. This necessitated dividing laser treatments over several sessions to give patients a break. Newer short duration pattern lasers apply energy over a fraction of the time and allow rapid completion of the laser treatment, usually in a single session with minimal patient discomfort. Computer technology and important digital images can be combined in one navigational laser system and enable the physician to pre-plan and target laser treatment to the different areas of the retina using customized settings. Navigated laser treatment also allows greater treatment accuracy and shorter treatment times.10 There was actually a recent paper which described how the retina specialist used 5G technology to program the navigated laser treatment to apply treatment on a patient some distance away.11
Subthreshold lasers are another promising innovation which utilize lower laser energy intensity to target tissues. Instead of PRP, where the aim is to destroy ischemic retinal tissues and decrease production of cytokines, subthreshold lasers stimulate release of restorative angiogenic factors to repair blood-retinal barriers. In contrast to standard laser treatment with subthreshold lasers, laser marks are not seen but a treatment effect can be observed. This method can reduce post-laser subfoveal scar extension and blind spot formation.
Lasers still are an important part of our range of treatments for DR. However, these treatments have limitations in the form of subpar visual outcomes.12-14 Dissatisfaction with these limitations led to a search for other treatment modalities.
The first biological drugs were actually developed in the 19th century when the diphtheria serum was produced in horses exposed to the diphtheria toxins. This led to a new era of pharmacotherapy using biologics, which have since been applied to a range of human diseases, including DR. With DME, laser treatment is unlikely to produce significant visual improvement. Thus, there was an unmet need for treatments that would not just resolve the edema, but also enhance visual function. Improved understanding of the central role of cytokines and growth factors in the breakdown of the blood-retina barriers and in neo-angiogenesis stimulated the development of pharmaceuticals and biologicals for the treatment of retinal diseases.4 The era of retinal pharmacotherapy began with intravitreal triamcinolone acetonide (IVTA) which has since been demonstrated to decrease central subfoveal thickness and improve visual acuity in eyes with DME.15 In the Philippines, we started performing IVTA in 2003 and I reported this technique at the Vitreo-Retina Society of the Philippines (VRSP) meeting in 2004 in Cavite, the birthplace of Dr. Romy Espiritu. The downsides of IVTA include transient visual loss, intraocular pressure (IOP) rise, cataract progression, and the occasional case of sterile and sometimes infectious endophthalmitis.15
The anti-VEGF era started with the introduction of pegaptanib sodium in the early 2000s. Pegaptanib selectively bound to and prevented the action of VEGF165, resulting in inhibition of both leakage from retinal blood vessels as well as decrease in neovascularization. While pegaptanib was predominantly used for wet age-related macular degeneration (AMD), when administered to eyes with DR, there was a significant dose-dependent improvement in visual acuity and concomitant reduction in central subfield thickness. Ranibizumab, a humanized monoclonal antibody fragment, later proved superior to pegaptanib. The game-changing improvements in visual activity that ranibizumab produced in the MARINA and ANCHOR studies for wet AMD and demonstrated in the RISE and RIDE studies for DME, of which Dr. Quan Dong Nguyen was the principal author, resulted in a paradigm shift.16 Henceforth, pharmacotherapeutics would be the standard of care for DME and wet AMD. Further studies like the RESTORE trial compared ranibizumab monotherapy against laser monotherapy and combined ranibizumab-laser treatment.17 The visual outcomes of ranibizumab alone, or combined with laser, were shown to be superior to laser therapy alone. Similar results were seen with aflibercept, a recombinant fusion protein for DME. An added benefit of aflibercept over ranibizumab was the possibility of bimonthly dosing, which decreased treatment burden.18 Ranibizumab and aflibercept are expensive anti-VEGF biologicals licensed for treatment of retinal diseases; as such, there is limited accessibility to these biologicals for patients in developing countries. Bevacizumab, licensed for use in treating colon cancer, can be administered at the fraction of the cost of ranibizumab and aflibercept. The BOLT study demonstrated the superiority of bevacizumab over laser treatment for DME.19
An important question is, “Are all anti-VEGFs created equally?”. Protocol T examined this question and compared the visual outcomes of patients receiving intravitreal ranibizumab, aflibercept or bevacizumab for DME. Overall, the eyes did well at 5 years whichever anti-VEGF was administered. Among the eyes that presented with moderately poor visual acuity though, ranibizumab and aflibercept seemed to do better than bevacizumab. But among the eyes presenting only mild visual decline, all three agents performed equally well. In Protocol T, over time, the number of injections decreased and by years 3 to 5, one third of patients did not need any more injections. Complication rates were low for all three agents.20
Inflammatory cytokines are linked to DR, so anti-inflammatory medications are theoretically beneficial. The MEAD study demonstrated the efficacy of intravitreal dexamethasone implant for improving visual outcomes and decreasing central subfield thickness among eyes with DME.21 Side effects included cataract progression. But among pseudophakic eyes, visual gains were maintained. An important benefit of dexamethasone implants is the reduced number of injections with eyes needing injection every few months instead of every few weeks. How do dexamethasone implants stack up against laser treatment? In 2021, we published the results of a multi-national trial which demonstrated that administering dexamethasone implant every five months was superior to focal laser treatment every three months in terms of improving visual acuity and reduction of central retinal thickness as well as total leakage area.22 Europeans believe that dexamethasone should be given even more frequently, every 3 to 4 months, in order to get the best results.23
Anti-VEGF therapy comes with a heavy treatment burden. In Protocol T, you can see on the treatment graph that each dot represents an injection per patient. You can see how many injections were given for all of these drugs in the first 2 years. We are always trying to look for a way to be more efficient and ease this treatment burden. Can a less stringent, customized treatment schedule work as well? In the Protocol I study, patients received either 4 monthly ranibizumab injections with prompt or deferred laser or triamcinolone acetonide or sham injection and prompt laser treatment. Thereafter, patients could receive deferred ranibizumab injections if the visual outcome was not yet considered a success or 20/20 or the retina was not yet dry. Well, it showed that ranibizumab with prompt laser or ranibizumab with deferred laser produced significant and sustained visual acuity improvements over time, compared to sham treatment.24 Giving delayed ranibizumab in initially sham-treated eyes produced improvements in visual acuity, but not achieving the level of those patients that received ranibizumab from the very start. Finally, triamcinolone initially produces impressive visual gains but with cataract onset, these gains were offset, resulting in visual decline. After being placed on ranibizumab, these eyes also showed some degree of catch up, but again, the gains were not as good as eyes that received ranibizumab from the very start.
Analysis of results of other studies shows that over time the number of injections needed to maintain vision decreased. In Protocol I, almost no injections were needed by year 5. This is good news for patients. These were the main learnings from Protocol I: (1) ranibizumab has better visual outcomes compared to laser; (2) adjunctive laser doesn’t enhance vision when combined with ranibizumab but may decrease the number of injections; (3) IVTA produces similar outcomes compared to ranibizumab in pseudophakic patients; (4) giving ranibizumab early improves vision more than giving ranibizumab late; (5) the number of ranibizumab injections decreases over time with majority of patients not requiring injections after year 3; (6) eyes that do not respond rapidly to ranibizumab may still display long-term benefit with continued therapy but may be less than those that improve immediately, and (7) intravitreal ranibizumab also improves DR severity scores. It also helps to control proliferative disease in about a third of eyes.24
Evidence-based DME treatment algorithms are now available. Dr. Sherman Valero, Dr. Jeffrey Lim, and I were fortunate to contribute to the Asian Consensus Guidelines.25 Essentially, these guidelines suggest that symptomatic DME should be treated with anti-VEGF medications as first line. If a patient has medical contraindications to anti-VEGF drugs, such as a recent stroke or heart attack, dexamethasone implant can be considered as first line. Dexamethasone implant can also be considered as first line in pseudophakic eyes as well as eyes that underwent previous vitrectomy.
Clinical trials tend to provide the ideal results, but can these results be replicated in the real world? The LUMINOUS study looked at ranibizumab outcomes among 500 diabetic eyes treated outside a trial protocol.26 In a real-world setting, the mean number of ranibizumab injections was far less than those given in a clinical trial and, not surprisingly, the visual improvement was also far less because of underdosing. We can also see that the patients with advanced disease and poor vision do not do as well as those patients who were treated early in the disease. What’s the best way to optimize anti-VEGF injections in order to gain the best outcomes? The best way, I think, should be to start strong and fast— just like a sprinter—with 3 monthly loading doses to maximize visual gains. Then we inject monthly as long as the patient’s vision is improving. Once you reach a ceiling effect in visual acuity, you start doing a treat-and-extend or treat-as-needed in the later phases—just like a marathon runner coasting to the finish line.
What’s the role of anti-VEGF drugs in proliferative disease? For 40 years now, the gold standard for treating proliferative DR has been PRP. But some are now advocating for anti-VEGF injections instead. In Protocol S, patients received either prompt laser treatment or ranibizumab or deferred laser treatment. At the start, eyes that received ranibizumab did better.27 At 5 years, visual outcomes were the same as eyes that underwent PRP. Cost-benefit analysis also demonstrated that anti- VEGF treatment for proliferative diseases is only cost-effective when there is simultaneous center- involved DME.28 In these cases, the anti-VEGF treatment may improve vision by controlling the proliferative disease and decreasing DME. If there is no DME, PRP is decidedly a more cost-effective treatment for proliferative disease.
This is a famous painting by Thomas Eakins of the Philadelphia physician, Dr. Samuel Gross. It was completed in 1875 for the Philadelphia centennial exposition. The painting was rejected by the organizing committee because of its graphic depiction of the blood and gore of real-life surgery. The painting was eventually placed in the Medical College of the Thomas Jefferson University. In 2006, the University decided to sell this painting to the National Gallery of Art in Washington DC for 68 million dollars, but the city of Philadelphia raised enough money to keep the painting in the Philadelphia Art Museum. Surgery is bloody and can be messy, but it is a valuable tool for managing many diseases such as DR. DR can be a bloody affair with vitreous hemorrhage (VH) and major cause of visual impairment. In 1985, the DR vitrectomy study established the following indications for diabetic surgery.29 We normally perform pars plana vitrectomy (PPV) for non-clearing VH and macular traction. The introduction of minimally-invasive PPV has enhanced the speed, success, and safety of diabetic surgery. In this era of pharmacotherapy, which is better for treating diabetic VH? Is it anti- VEGF or invasive PPV? A recently completed study randomized patients with VH to monthly aflibercept injection or PPV with photocoagulation. The visual outcomes were equal for aflibercept- and PPV- treated eyes.30 Other studies further demonstrated the cost benefit of the different treatment modalities.31 In short, anti-VEGF and laser treatment achieved the same improvement in quality of life at the same cost, but intravitreal anti-VEGF was at least three times more costly than the other two modalities. This result suggested a limited role for intravitreal anti-VEGF treatments for proliferative DR and that laser treatment should still be considered.
What happens when we combine treatment modalities? For example, can we combine laser with pharmacotherapy? The PROTEUS study determined that combining PRP with intravitreal ranibizumab led to more rapid reduction of neo-vascularization and fewer laser sessions than laser treatment alone.32 Visual and anatomic outcomes were the same. If there is DME, adding anti-VEGF leads to better vision and fewer laser sessions. How about combining laser treatment with surgery? Applying PRP to at least 25% of the retina before performing PPV seems to improve visual outcomes.33 So whenever possible, put some laser shots in before doing vitrectomy. Is there a role for combining pharmacotherapy and surgery? A meta-analysis demonstrated decreased incidents of postoperative VH if anti-VEGF drug is applied shortly before or at the end of PPV.34 There are many case series that recommend putting in anti-VEGF a week or two before vitrectomy in order to reduce intraoperative bleeding and facilitate PPV. There is also anecdotal evidence that putting anti-VEGF at the end of the surgery may prevent postoperative vitreous hemorrhage and facilitate visual recovery.
This painting depicts Saint Peter leaving Rome to escape Christian persecution. On the way, he meets Jesus carrying a cross, and Peter asks him, “Quo vadis, dominae?”(“Where are you going, Lord?”), and Jesus answers, “I’m going back to Rome to be crucified again.” Peter, feeling guilty, turns around and goes back to Rome to continue his evangelical work and to his own eventual crucifixion.
We have so far reviewed a lot of miraculous progress in the management of DR. Where do we go from here? There are several exciting areas of development in the fields of imaging, data analytics, and pharmacology that will change our practices in the near future. All of these will bring us to a new era of personalized medicine. Medicine has entered a new era that utilizes data and artificial intelligence (AI) to improve health outcomes. For example, AI can predict diabetic disease progression based on imaging and other biomarkers.35 The potential applications of this technology are enormous. Recall that there are 100 million diabetics around the world that will require periodic screening for retinopathy. AI has the potential to automate this process and lessen the workload of health care systems.36,37 At our practice, we collaborate with non-governmental organizations to rapidly screen diabetic patients outside of Metro Manila. Previously, it would take a whole day and a staff of four to screen a hundred patients going through dilation and indirect ophthalmoscopy. Using a portable fundus camera linked to AI cloud software, a single technician can now screen those hundred patients in half a day. How accurate is this system? We found it to be very accurate. On the top here, you can see eyes without retinopathy that were graded negative retinopathy by the software, so this is correct (Figures 1A and 1B). On the bottom, you can see eyes without retinopathy but were graded to have positive retinopathy because of these reflections on the retina (Figures 1C and 1D). Here, we see retinas with cotton-wool spots and dot hemorrhages which were graded correctly by the system as having positive retinopathy (Figures 1E and F). With this AI-powered system, we approach 90% sensitivity and 97% specificity. Importantly, patients that were erroneously labeled as positive for retinopathy more often than not had some other retinal pathology that needed a further evaluation, such as macular degeneration; and patients under-diagnosed as negative retinopathy had a non-clinically significant diabetic retinal disease that required no intervention. Patients in remote areas can avail of these screening programs and only have to travel to eye clinics when they actually have a disease that needs to be managed.

Aside from detection and grading of disease severity, AI can also use date to predict disease progreassion.35 In this study a deep learning algorithm was fed patient data, such as demographic data, laboratory data, and was also fed data as to which eyes evetually developed DR progression. Over time, the AI software became more accurate predicting which eyes experience worsening of disease.
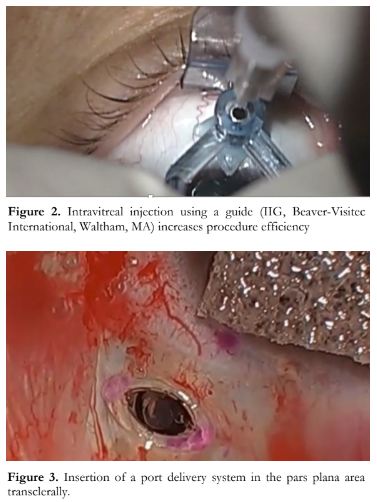
Because of the high treatment burdens with large numbers of patients needing regular injections, there is also a need to streamline the injection process. We recently reported on using interventional injection guides in order to facilitate the injection process.38 So here in this video, you can see how the injection guides (Figure 2) can be used to guide and smoothen the injection process. This system also results in less subconjunctival hemorrhage, making the injection experience more comfortable and actually encourages treatment compliance. Another way to lessen treatment burden is to have a refillable drug reservoir. Instead of monthly intravitreal shots, retina surgeons would then just have to fill up the port-delivery system with the drug every few months. Here you can see the port delivery system being easily installed in the pars plana area trans-sclerally (Figure 3). And here you can see the process of injecting. You can identify the entrance of the port delivery system and then just simply inject the drug into it and over time, this system will release the drug in a sustained manner. It’s like filling your gas tank every few weeks instead of every day. The Ladder and Archway trials investigated the results of this port delivery system and found that you can get similar results refilling the PORT delivery system every few months, as injecting the eye with ranibizumab every month.39 This is an exciting development and it will greatly decrease the present treatment burden for patients and doctors.
Promising new medicines are also in the pipeline. Brolucizumab is already registered for treatment of wet AMD in the US and Europe. The Phase III KITE study has demonstrated the non- inferiority of brolucizumab to aflibercept in terms of visual outcomes, and superiority in terms of reducing central subfield thickness.40 Of note, half of brolucizumab-treated eyes were able to maintain visual gains with every 12-week dosing. Faricimab is another very interesting molecule which simultaneously inhibits VEGF and angiopoietin, which leads to improved vascular stability and reduces retinal inflammation. Phase II studies have demonstrated rapid and robust visual gains over current treatments.41
The holy grail of personalized medicine is gene therapy. Potential targets for gene therapy include downregulation of VEGF by switching off specific genes needed to produce VEGF and angiopoietin, reduction of oxidative stress by promoting the production of superoxide dismutase, and targeting genes of ACE-2 angiotensin or MAS receptors. For gene therapy to be accepted, the delivery method should also be easy. Currently, you have to do surgery and inject the vector subretinally, a very complex and complicated system. Hopefully in the future, we will be able to deliver the vectors with a simple transscleral intravitreal injection and then gene therapy would really take off. It’s not a matter of if, but when personalized medicine will become available. Combining big patient data, patient outcomes, digital imaging, artificial intelligence will be able to help perform outcomes analysis and be able to predict which patients will do best with what treatment and when treatment should be started.41 In this age of climate change and pandemics, it’s our duty as physicians to be open-minded and keep an eye on the future and adopt these innovative technologies that will be able to provide targeted treatments and the most cost-effective outcomes, giving our patients the best quality of life. I know that my mentor, the late Dr. Romeo B. Espiritu, would approve with a twinkle in his eye.
In summary, we are now in a golden age of treatments for DR. The emergence of novel transformative technologies holds promise for expanding access to diabetic screening and treatment, individualizing patient care, and maximizing cost effectiveness and visual outcomes. We will be well-equipped to meet the challenges ahead.
In closing, let us remember our past but work towards a better future. Thank you again to the VRSP for the privilege of delivering the 2021 Romeo B. Espiritu Memorial Lecture. I would like to thank my many mentors, colleagues, batchmates, and patients, for helping me become a better physician. And special thanks to my family for their sacrifices, understanding, and support all these years. Maraming salamat po.
REFERENCES
- International Diabetes Federation. IDF Diabetes Atlas, 8h edn. Brussels, Belgium. 2017: https://www.diabetesatlas.org (accessed November 1, 2021).
- Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556-64.
- Tremolada G, Del Turco C, Lattanzio R, et al. The role of angiogenesis in the development of proliferative diabetic retinopathy: impact of intravitreal anti-VEGF treatment. Exp Diabetes Res. 2012;2012:728325.
- Kusuhara S, Fukushima Y, Ogura S, et al. Pathophysiology of Diabetic Retinopathy: The Old and the New. Diabetes Metab J. 2018;42(5):364-376.
- Ashraf M, Cavallerano JD, Sun JK, et al. Ultrawide Field Imaging in Diabetic Retinopathy: Exploring the Role of Quantitative Metrics. J Clin Med. 2021;10(15):3300.
- The Diabetic Retinopathy Study Research Group. A Modification of the Airlie House Classification of Diabetic Retinopathy. DRS report #7. Invest Ophthalmol Vis Sci. 1981;21:210–26.
- The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy: Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology. 1981;88(7):583-600.
- Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991;98(5 Suppl):741-56.
- Tababat-Khani P, Bengtsson B, Agardh E. Effects of focal/grid laser treatment on the central visual field in diabetic macular oedema: a 2-year follow-up study. Acta Ophthalmol. 2016;94(3):240-5.
- Chhablani J, Kozak I, Barteselli G, El-Emam S. A novel navigated laser system brings new efficacy to the treatment of retinovascular disorders. Oman J Ophthalmol. 2013;6(1):18-22.
- Chen H, Pan X, Yang J, et al. Application of 5G Technology to Conduct Real-Time Teleretinal Laser Photocoagulation for the Treatment of Diabetic Retinopathy. JAMA ophthalmology 2021;139(9):975–982.
- Lee CM, Olk RJ. Modified grid laser photocoagulation for diffuse diabetic macular edema. Long-term visual results. Ophthalmology. 1991;98(10):1594-602.
- Sahid MJ, Ahmad F, Asif M, Sultan MN. Visual outcome in diabetic macular edema after grid laser treatment. Professional Med J. 2016;23(4):478-83.
- Jyothi S, Sivaprasad S. Five-year visual outcome following laser photocoagulation of diabetic macular oedema. Eye (Lond). 2011;25(7):851-8.
- Sutter FK, Simpson JM, Gillies MC. Intravitreal triamcinolone for diabetic macular edema that persists after laser treatment: three-month efficacy and safety results of a prospective, randomized, double-masked, placebo-controlled clinical trial. Ophthalmology. 2004;111(11):2044-9.
- Nguyen QD, Brown DM, Marcus DM, et al.; RISE and RIDE Research Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789-801.
- Mitchell P, Bandello F, Schmidt-Erfurth U, et al.; RESTORE study group. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for dbetic macular edema. Ophthalmology. 2011;118(4):615-25.
- Heier JS, Korobelnik JF, Brown DM, et al. Intravitreal Aflibercept for Diabetic Macular Edema: 148-Week Results from the VISTA and VIVID Studies. Ophthalmology. 2016;123(11):2376-2385.
- Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117(6):1078-1086.
- Glassman AR, Wells JA 3rd, Josic K, et al. Five-Year Outcomes after Initial Aflibercept, Bevacizumab, or Ranibizumab Treatment for Diabetic Macular Edema (Protocol T Extension Study). Ophthalmology. 2020;127(9):1201-1210.
- Boyer DS, Yoon YH, Belfort R Jr, et al.; Ozurdex MEAD Study Group. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904- 14.
- Wei W, Chen Y, Hu B, et al. Multicenter, Prospective, Randomized Study of Dexamethasone Intravitreal Implant in Patients with Center-Involved Diabetic Macular Edema in the Asia-Pacific Region. Clin Ophthalmol. 2021;15:4097- 4108.
- Rosenblatt A, Udaondo P, Cunha-Vaz J, et al.; ARTES Study Group. A Collaborative Retrospective Study on the Efficacy and Safety of Intravitreal Dexamethasone Implant (Ozurdex) in Patients with Diabetic Macular Edema: The European DME Registry Study. Ophthalmology. 2020;127(3):377-393.
- Bressler SB, Glassman AR, Almukhtar T, et al.; Diabetic Retinopathy Clinical Research Network. Five-Year Outcomes of Ranibizumab With Prompt or Deferred Laser Versus Laser or Triamcinolone Plus Deferred Ranibizumab for Diabetic Macular Edema. Am J Ophthalmol. 2016;164:57-68.
- Chhablani J, Wong K, Tan GS, et al. Diabetic Macular Edema Management in Asian Population: Expert Panel Consensus Guidelines. Asia Pac J Ophthalmol (Phila). 2020 Sep-Oct;9(5):426-434.
- Mitchell P, Sheidow TG, Farah ME; LUMINOUS study investigators. Effectiveness and safety of ranibizumab 0.5 mg in treatment-naïve patients with diabetic macular edema: Results from the real-world global LUMINOUS study. PLoS One. 2020;15(6):e0233595.
- Gross JG, Glassman AR, Liu D, et al.; Diabetic Retinopathy Clinical Research Network. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2018;136(10):1138-1148.
- Hutton DW, Stein JD, Glassman AR; DRCR Retina Network. Five-Year Cost-effectiveness of Intravitreous Ranibizumab Therapy vs Panretinal Photocoagulation for Treating Proliferative Diabetic Retinopathy: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2019;137(12):1424-1432.
- The Diabetic Retinopathy Vitrectomy Study Research Group. Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Two-year results of a randomized trial. Diabetic Retinopathy Vitrectomy Study report 2. Arch Ophthalmol. 1985;103(11):1644-52.
- Antoszyk AN, Glassman AR, Beaulieu WT, et al.; DRCR Retina Network. Effect of Intravitreous Aflibercept vs Vitrectomy With Panretinal Photocoagulation on Visual Acuity in Patients With Vitreous Hemorrhage From Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA. 2020;324(23):2383-2395.
- Lin J, Chang JS, Yannuzzi NA, Smiddy WE. Cost Evaluation of Early Vitrectomy versus Panretinal Photocoagulation and Intravitreal Ranibizumab for Proliferative Diabetic Retinopathy. Ophthalmology. 2018;125(9):1393-1400.
- Figueira J, Fletcher E, Massin P, et al.; EVICR.net Study Group. Ranibizumab Plus Panretinal Photocoagulation versus Panretinal Photocoagulation Alone for High-Risk Proliferative Diabetic Retinopathy (PROTEUS Study). Ophthalmology. 2018;125(5):691-700.
- Thompson JT, de Bustros S, Michels RG, Rice TA. Results and prognostic factors in vitrectomy for diabetic traction- rhegmatogenous retinal detachment. Arch Ophthalmol. 1987;105(4):503-7.
- Goodart R, Blankenship G. Panretinal photocoagulation influence on virectomy results for complications of diabetic retinopathy. Ophthalmology. 1980;87(3):183-8.
- Arcadu F, Benmansour F, Maunz A, et al. Deep learning algorithm predicts diabetic retinopathy progression in individual patients. NPJ Digit Med. 2019;2:92.
- Li F, Liu Z, Chen H, et al. Automatic Detection of Diabetic Retinopathy in Retinal Fundus Photographs Based on Deep Learning Algorithm. Transl Vis Sci Technol. 2019;8(6):4.
- Le D, Alam M, Yao CK, et al. Transfer Learning for Automated OCTA Detection of Diabetic Retinopathy. Transl Vis Sci Technol. 2020;9(2):35.
- Uy HS, Artiaga JCM. Comparison of Two Different Intravitreal Injection Techniques. Clin Ophthalmol. 2021;15:2383-2389.
- Khanani AM, Callanan D, Dreyer R, et al. End-of-Study Results for the Ladder Phase 2 Trial of the Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology Retina. 2021; 5(3)775- 787.
- Garweg JG. A Randomized, Double-Masked, Multicenter, Phase III Study Assessing the Efficacy and Safety of Brolucizumab versus Aflibercept in Patients with Visual Impairment due to Diabetic Macular Edema (KITE). Klin Monbl Augenheilkd. 2020;237(4):450-453.
- Sahni J, Patel SS, Dugel PU, et al. Simultaneous Inhibition of Angiopoietin-2 and Vascular Endothelial Growth Factor-A with Faricimab in Diabetic Macular Edema: BOULEVARD Phase 2 Randomized Trial. Ophthalmology. 2019;126(8):1155-1170.
- Zhang S, Bamakan SMH, Qu Q, Li S, Learning for Personalized Medicine: A Comprehensive Review From a Deep Learning Perspective. IEEE Reviews in Biomedical Engineering. 2019;12: 194-208.

