Postoperative Safety Outcomes in Patients Undergoing Routine Phacoemulsification Cataract Surgery with Intraoperative Intracameral Injection of Preservative-Free Moxifloxacin versus Levofloxacin
Franz Marie Cruz1,2, Harvey S. Uy1,2, Carlo Josemaria Rubio1, Pik Sha Chan1
1Peregrine Eye and Laser Institute, Makati City, Philippines
2Department of Ophthalmology and Visual Sciences, University of the Philippines – College of Medicine, Philippine General Hospital, Manila, Philippines
Correspondence: Harvey S. Uy
Peregrine Eye and Laser Institute, Morning Star Center , 347 Jupiter Street, Bel-Air, Makati City, Philippines, 1209
Tel: + 63 2 8890 0115, Fax: +63 2 8241 1153
Email: harveyuy@yahoo.com
Disclosure: Harvey S. Uy has received research funding from Santen, Inc. The other authors report no conflicts of interest in this work.
Introduction
Cataract surgery is the most commonly performed ocular surgery in the world. Endophthalmitis, a rare but devastating sight- threatening postoperative complication of intraocular surgery, is characterized by microbial- induced inflammation within the intraocular cavity. Approximately 90% of endophthalmitis cases occur following cataract surgery with an incidence rate ranging from 0.08 to 0.7%.1,2
Aseptic technique, preoperative topical povidone-iodine, and antibiotic prophylaxis are crucial for endophthalmitis prevention.3 A variety of measures can be employed as chemoprophylaxis against bacterial endophthalmitis including topical or subconjunctival antibiotics administered before, during or after surgery. Unsettled issues include questions like which antibiotic and/or route of administration would provide the most effective and safest protection. Traditionally, topical antibiotics are administered before and/or after surgery and subconjunctival injection of antibiotics is given at the end of the surgery. Recent evidence from one large clinical trial and several observational studies supports the safety and efficacy of intracameral antibiotics for preventing postoperative endophthalmitis.4-8 In a survey, the American Society of Cataract and Refractive Surgery (ASCRS) reported that 50% of 1,147 global respondents were injecting intracameral antibiotics at the conclusion of surgery.9
Cefuroxime, a second-generation cephalosporin, was first used as an intracameral prophylaxis in the late 1990s.10 The safety and efficacy of intracameral cefuroxime were further established by the European Society of Cataract and Refractive Surgeons (ESCRS) in its 2007 landmark study which demonstrated a five-fold reduction of endophthalmitis rates following intracameral cefuroxime administration with or without topical levofloxacin use.11 The lowest incidence of endophthalmitis was recorded among patients who received both topical levofloxacin and intracameral cefuroxime.11 A 2015 retrospective study reported that 112 cases of endophthalmitis developed among 480,104 cataract surgeries (0.023%) when topical antibiotics were used, compared to no cases among 25,920 eyes (0%) that received intracameral cefuroxime.12 Other studies have corroborated the efficacy of intracameral cefuroxime; however, there is evidence of gaps in antimicrobial coverage and emerging resistance to cefuroxime.10,13-16 Cefuroxime also carries a risk of hypersensitivity reaction especially in patients with a history of allergy to penicillin or cephalosporins.13 Cefuroxime is also only available as a systemic preparation and requires reconstitution which brings up potential concerns regarding ocular toxicity resulting from dilution errors.13,17
Recent years have witnessed the increased utilization of fluoroquinolones, such as besifloxacin, gatifloxacin, levofloxacin and moxifloxacin, for prevention of postoperative infection. Levofloxacin and moxifloxacin are commercially available as preservative-free eye drops and are approved for the treatment of bacterial conjunctivitis. They are broad-spectrum antibiotics that are active against many gram-positive and gram-negative bacteria, including the majority of the causative organisms of post-cataract surgery endophthalmitis.13 Both fluoroquinolones act by inhibiting bacterial DNA gyrase and topoisomerase IV.18 An additional advantage of moxifloxacin and levofloxacin over cefuroxime is their drug kinetics. While the bactericidal activity of cefuroxime is time-dependent, moxifloxacin and levofloxacin are concentration-dependent which, if given as a bolus at the end of the cataract surgery, may lead to a more effective eradication of bacteria.6,19 We elected to study these two particular drugs because they are both widely distributed and are available as preservative-free eye drops. This means the drug can be directly extracted from the eye drop bottle and placed into the anterior chamber without the need for reconstitution or other processing. This makes for an efficient method of preparing and delivering an anti- bacterial regimen at the end of cataract surgery.
Early data support the safety and efficacy of both preservative-free moxifloxacin and levofloxacin as intracameral chemoprophylaxis.4,7,17,19-26 However, there are no studies that compare these two antibiotics head-to-head. This study was designed to evaluate and compare ocular safety outcomes associated with the use of either intracameral moxifloxacin or levofloxacin in patients undergoing routine phacoemulsification.
Subjects and Methods
Study Design and Preoperative Assessments
This was a single-center, prospective, double- masked, randomized, interventional study which enrolled patients older than 21 years of age, with visually-significant cataracts who underwent uncomplicated phacoemulsification and in-the-bag intraocular lens (IOL) at the Peregrine Eye and Laser Institute, Makati, Philippines from January 2 to April 28, 2018. Only one study eye per patient was included in the study. Patients who had other ocular pathologies such as corneal opacities, corneal dystrophies, glaucoma, uveitis, retinopathy, optic neuropathy, concomitant infection (blepharitis, hordeolum or conjunctivitis) or uncontrolled systemic disease were excluded from the study. Eligible patients were provided a copy of the study protocol during the preoperative clinic visit and an explanation of the study protocol and processes. Those who voluntarily provided consent were enrolled in the study. The study protocol and informed consent forms were approved by the Peregrine Eye and Laser Institute Institutional Review Board (PELI-IRB). All patients provided signed written informed consent for participation in this study. The study was performed in accordance with Good Clinical Practices and the Declaration of Helsinki.
The preoperative evaluation included history-taking, review of systems, visual acuity testing using a Snellen chart, Goldman applanation tonometry, slit-lamp biomicroscopy (SL-D7, Topcon, Tokyo, Japan), and dilated retinal examination. Cataract density was graded using Lens Opacities Classification System III by a certified examiner.
Each study eye underwent the following preoperative measurements: endothelial cell density (ECD) using specular microscopy (CellChek XL, Konan Medical, Irvine, CA, USA); assessment of the central corneal thickness (CCT) using the Scheimpflug imaging system (Pentacam HR, Oculus, Wetzlar, Germany); and measurement of the central retinal thickness (CRT) and macular volume (MV) using spectral-domain optical coherence tomography (Cirrus HD-OCT, Carl Zeiss Meditec, Jena, Germany). IOL calculation was carried out using optical biometry (IOLMaster 700, Carl Zeiss Meditec, Jena, Germany).
The study eyes were randomly assigned to 1 of 2 treatment groups: the 0.5% moxifloxacin (M) and 0.5% levofloxacin (L) groups. Study participants were masked to their assigned treatment group.
Surgical Technique
As preoperative preparation, proparacaine (Alcaine, Alcon Laboratories, Fort Worth, TX, USA) and 5% povidone iodine solution were placed into the conjunctival cul-de-sac. Povidone iodine 10% paint was applied to the eyelids and periorbital skin. Standard, temporal-approach phacoemulsification through a 2.2mm clear corneal incision was performed by a single surgeon (HSU) using chopping techniques and a single phacoemulsification machine (Centurion, Alcon Surgical, Fort Worth, TX, USA). A single-use ophthalmic viscoelastic device (OVD) (Discovisc, Alcon Surgical) was utilized for all cases. Each eye was implanted with one (of a variety) of single-piece acrylic IOLs, which were placed into the capsular bag using an assisted injection technique. This was followed by aspiration of all remaining OVD and reformation of the anterior chamber (AC) with balanced saline solution injected via side port. After securing a stable and formed AC, 0.5mg in 0.1ml of the intracameral antibiotic in the form of 0.5% moxifloxacin (Vigamox, Alcon Laboratories) or unpreserved 0.5% levofloxacin (Oftaquix, Santen, Osaka, Japan) was injected via side port. The eyelid retractors were removed and a drop of the same antibiotic, plus 1% prednisolone acetate (Pred Forte, Allergan Inc., Madison, NJ, USA), was instilled into the conjunctival cul-de-sac.
Post-Operative Medications
All patients received 1% prednisolone acetate (Pred Forte), 1 drop 4x a day on the study eye for 1 month. Independent of the treatment group assignment, all eyes received 0.3% gatifloxacin ophthalmic suspension drops (Zymar, Allergan Sales, Waco, TX, USA) administered 4x a day on the study eye for 2 weeks in order to maintain postoperative masking.
Post-operative Evaluation and Study Outcomes
Follow-up eye examinations were performed at postoperative day 1, week 1 and month 1. These included repeat measurements of ECD, CCT, CRT and MV by the same technicians who were masked to the treatment group assignments. Primary study outcome measures included ocular safety parameters namely ECD, CCT, CRT and MV. Secondary study outcome measures included study-related adverse events.
Statistical Analyses
Statistical analyses were performed with Graphpad Prism statistical software version 6.01 (Graph Pad Software, La Jolla, CA, USA). A paired t-test was applied to test the significance of differences between means of the two groups. A P value <0.05 was considered to indicate a statistically significant difference.
Results
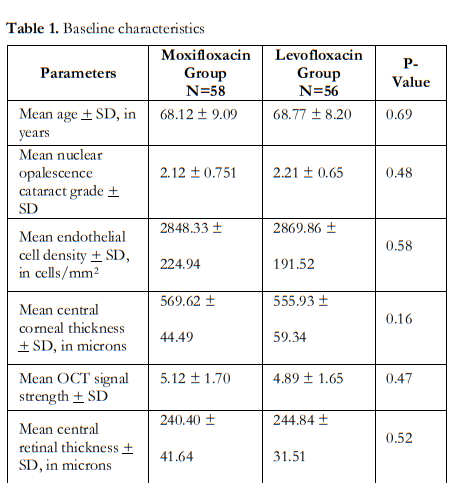
One-hundred-and-fourteen (114) patients were enrolled in the study with mean age of 68 ± 9 (range 50 to 86) years. Seventy-four (74 or 65%) were female. Fifty-eight (58) study eyes (51%) were in the moxifloxacin (M) group, while 56 (49%) were in the levofloxacin (L) group. Table 1 shows similar baseline parameters between the 2 groups.
Repeat measurements taken during the post- operative visits day 1, week 1 and month 1 are shown in Table 2. Between-group comparisons showed no significant differences in the means of ECD, CCT, CRT and MV at all study visits.

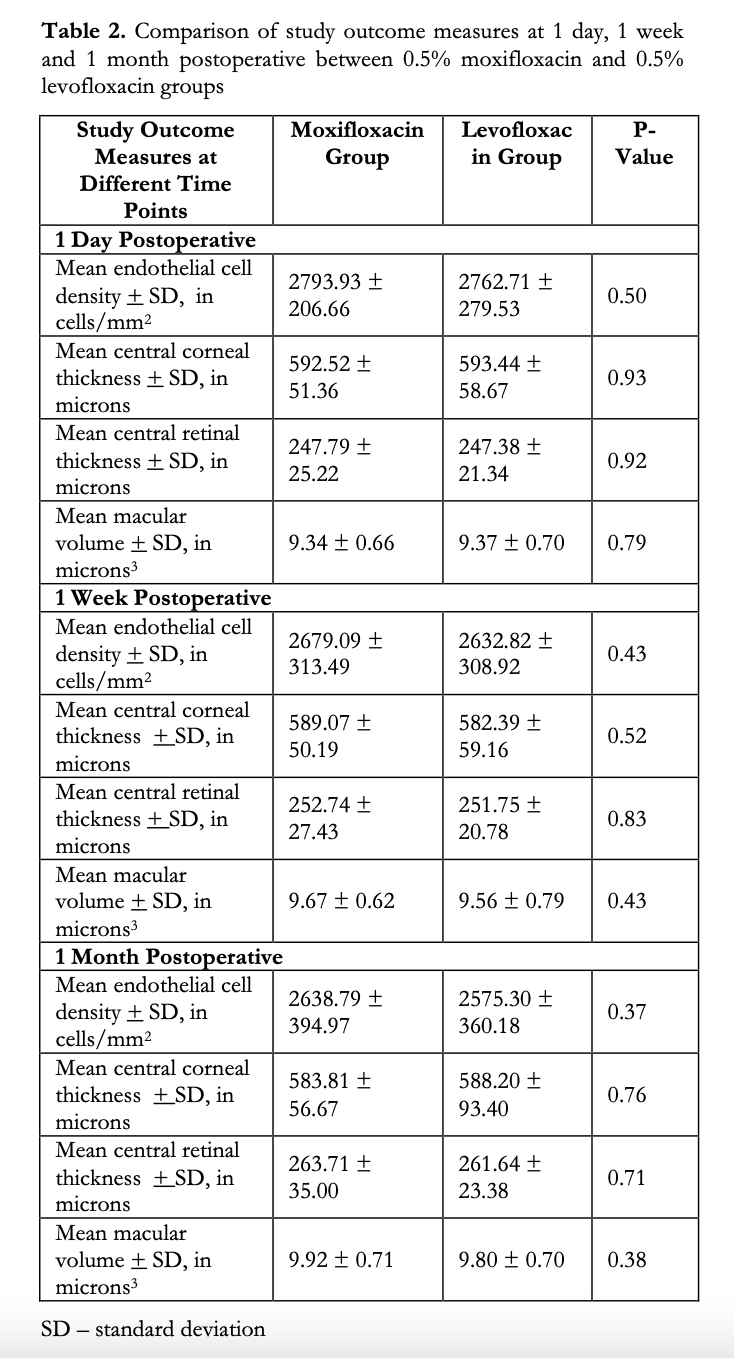
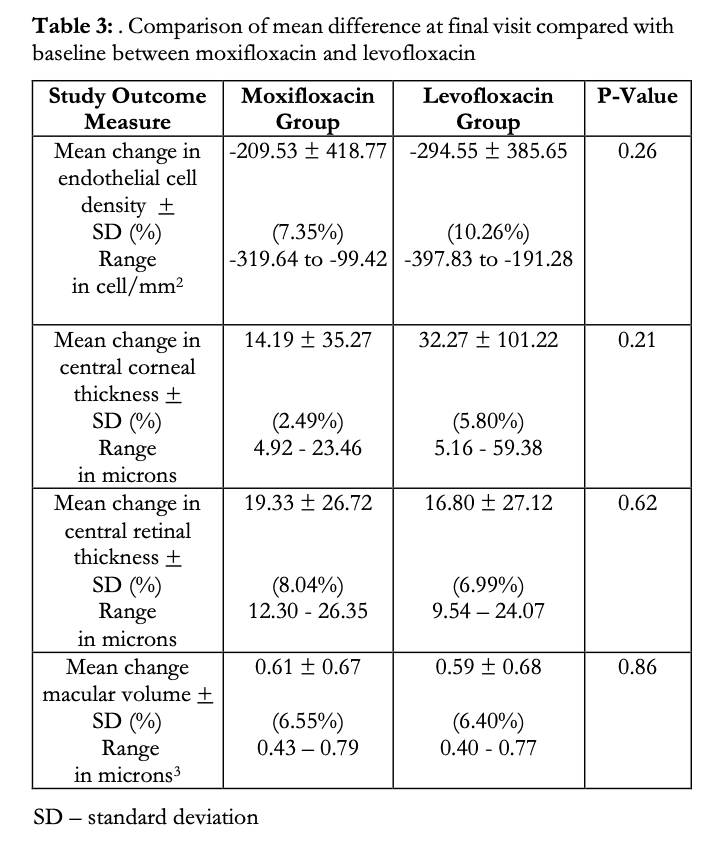
A between-group comparison was made on the mean change in all 4 study parameters from baseline to final visit (Table 3). A decrease in the mean ECD was observed in both study groups (M: -209.53 vs L: -294.55 cells/mm2). These correspond to a 7.35 and 10.26% decrease from baseline ECD for M and L groups, respectively, however the difference between the mean changes of the M and L groups was not significant (p=0.262). Likewise, although there was a slight increase in the mean CCT in both groups (M: 14.19 vs L: 32.27μm), the difference was statistically insignificant (p=0.21). Slight increases in the means of CRT and MV were also observed in both groups, but between-group analysis showed no statistical difference (M:19.33 vs L:16.80μm, p=0.62, and M:0.61 and L:0.59μm3, p=0.86 for CRT and RV, respectively).
Lastly, there were no study-related adverse events observed during the course of the study. There were no cases of intraocular hypertension, cystoid macular edema, toxic anterior segment syndrome or endophthalmitis.
Discussion
Moxifloxacin, a fourth-generation fluoroquinolone, covers a broad spectrum of organisms, making it effective in preventing post-surgical infection.26 Levofloxacin, a broad-spectrum third-generation fluoroquinolone, has recently emerged as another safe alternative in preventing post-surgical infection.17 Previous studies have found moxifloxacin to be both safe and effective for preventing experimental endophthalmitis. A large retrospective study involving more than 600,000 intraocular surgeries compared the postoperative endophthalmitis rate before and after initiation of intracameral moxifloxacin prophylaxis.24 Study findings demonstrated 3.5 fold reduction in overall endophthalmitis rate when routine intracameral moxifloxacin prophylaxis was used.24 In addition, another 2017 study involving 3,680 eyes of 1,913 patients found a 7.3-fold lower ratio of endophthalmitis following intracameral moxifloxacin.19 A meta-analysis by Bowen and colleagues concluded that intracameral cefuroxime and moxifloxacin are both efficacious and cost-effective in reducing the incidence of postsurgical endophthalmitis.27
In 2007, a study by Espiritu and others demonstrated the safety of intracameral moxifloxacin administered at the end of the surgery.20 The authors found it non-toxic to the parameters of visual rehabilitation, anterior chamber reaction, pachymetry and corneal endothelial cell density. Since then, several other studies have shown further evidence on the safety of intracameral moxifloxacin in human eyes undergoing cataract surgery.4,7,21,23,25
On the other hand, there is only one published study on the ocular safety of intracameral injection of unpreserved levofloxacin post cataract surgery. In 2017, Espiritu and Bolinao investigated the ocular safety of intracameral levofloxacin in 50 eyes of 50 patients who underwent phacoemulsification and IOL implantation; they reported no safety concerns in terms of visual acuity, endothelial cell count, degree of anterior segment inflammation and central foveal thickness associated with intracameral injection of levofloxacin 0.5% prophylactically following cataract surgery.17
These results are in line with an earlier preclinical study by Kim et al., where the authors compared the toxicity of intracameral moxifloxacin and levofloxacin, along with cefazolin in rabbit eyes. They concluded that intracameral injections of all three studied antibiotics were safe and non-toxic for surgical prophylaxis.22
This study compared postoperative outcomes of intracameral administration of preservative-free moxifloxacin and levofloxacin. We found no significant differences in postoperative ocular safety outcomes between the two treatments groups following intracameral prophylactic use of either agent. We assessed postoperative changes in ECD, corneal thickness, macular volume and CRT to determine the safety of intracameral moxifloxacin versus levofloxacin on the anterior and posterior segments of the eye. In terms of changes in the ECD, our study findings show a 7.35 and 10.26% decrease at 1 month postoperative, compared to baseline in the M and L groups, respectively. This is within the range of reported reduction in ECD (4.8-11%) at 1 month following phacoemulsification.28-31
Central corneal thickness typically increases immediately following phacoemulsification and reflects the function of the remaining endothelial cells.31,32 However, irrespective of the number of endothelial cell loss, the central corneal thickness is reported to return to its preoperative value by 3-12 months after surgery.32,33 Compared to baseline, central corneal thickness in the M and L groups was still 2.49 and 5.80% thicker than baseline, respectively, at 1 month after surgery. Our short follow-up duration prevented further observation of central corneal thickness if indeed there was return to the preoperative state.
Posterior segment effects of phacoemulsification have been measured using changes in CRT and retinal volume. Release of pro-inflammatory mediators, light damage and vitreoretinal traction are some factors hypothesized to contribute to the breakdown in the blood-retinal barrier, resulting in increased retinal thickness and, when severe, cystoid macular edema. Macular thickness and volume have been reported to progressively increase up to 6 months following cataract surgery.34-37 These increasing trends in CRT and retinal volume were also observed in this study. However, qualitative examination of our OCT images did not show development of intraretinal cysts that would suggest cystoid macular edema.
Despite changes in the corneal and retinal parameters used in the study, these changes were not unexpected and were consistent with several previous studies. In addition, no significant differences were observed in the ocular parameters between the 2 treatment groups at baseline and at 3 postoperative visits. Our results suggest that intracameral application of preservative-free levofloxacin and moxifloxacin is equally safe when used as postoperative antibacterial prophylaxis among eyes undergoing cataract surgery. Their similar safety profiles may be attributed to near identical chemical properties. Oftaquix (based on levofloxacin) has a pH of 6.2-6.8 and osmolality of 300 mOsm/L, while the values for Vigamox (based on moxifloxacin) are 6.8 and 290 mOSm/L, respectively.22
Lastly, we observed no study-related adverse events during the course of the study. No study eye developed elevated intraocular pressure, corneal decompensation, cystoid macular edema, toxic anterior segment syndrome or infectious endophthalmitis. It is recognized, however, that this study was not originally designed to detect the rare occurrence of post-cataract surgery infectious endophthalmitis.
A key strength of our research is the prospective study design. Randomization and masking were employed to minimize the risk of bias. This is also the first study to compare intracameral preservative-free moxifloxacin and levofloxacin head-to-head using 4 standard ocular safety parameters. We assessed the possible ocular toxic effects of the 2 drugs, not only on the anterior segment, but also on the retina. Our study findings add to the growing evidence on ocular safety of the off-label use of intracameral injection of preservative-free moxifloxacin and levofloxacin. The main limitations of our study were the sample size and short duration of follow-up. This study also did not assess other measures of corneal endothelial damage, such as hexagonality and corneal volume, nor was it sufficiently powered to evaluate the efficacy of both drugs in the prevention of endophthalmitis. Therefore, we propose larger studies with longer follow-up duration to detect possible differences not captured within our study population. An area for further research is comparison of cost effectiveness of postoperative antibiotic eye drops versus intracameral antibiotics alone.
In summary, intracameral moxifloxacin and levofloxacin appear to have similar safety outcomes when used as antibacterial prophylaxis among eyes undergoing cataract surgery. Both fluoroquinolone-based agents are potentially suitable options for endophthalmitis chemoprophylaxis. Larger scale studies are needed for further efficacy analysis.
Acknowledgments
The authors would like to thank Rheamel Navarro for assistance with statistical analyses.
REFERENCES
- Kernt M, Kampik A. Endophthalmitis: Pathogenesis, clinical presentation, management, and perspectives. Clin Ophthalmol. 2010;4:121–135.
- Safneck JR. Endophthalmitis: A review of recent trends. Saudi J Ophthalmol. 2012;26:181-189.
- Schwartz SG, Flynn HW. Update on the prevention and treatment of endophthalmitis. Expert Rev Ophthalmol. 2014;9:425-430.
- Koktekir BE, Aslan BS. Safety of prophylactic intracameral moxifloxacin use in cataract surgery. J Ocul Pharmacol Ther. 2012;28:278-82.
- Behndig A, Cochener B, Güell JL, et al. Endophthalmitis prophylaxis in cataract surgery: Overview of current practice patterns in 9 European countries. J Cataract Refract Surgery. 2013;39:1421-1431.
- Galvis V, Tello A, Sanchez MA, Camacho PA. Cohort study of intracameral moxifloxacin in postoperative
endophthalmitis prophylaxis. Ophthalmol Eye Dis. 2014;6:1-4. - Matsuura K, Miyoshi T, Suto C, et al. Efficacy and safety of prophylactic intracameral moxifloxacin in Japan. J Cataract Refract Surg. 2013;39:1702-6.
- Tranos P, Dervenis N, Vakalis AN, et al. Current Perspectives of Prophylaxis and Management of Acute
Infective Endophthalmitis. Adv Ther. 2016;33:727-746. - Chang DF, Braga-Mele R, Henderson BA, et al. Antibiotic prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2014 ASCRS member survey. J Cataract Refract Surg. 2015;41:1300–1305.
- Montan PG, Wejde G, Koranyi G, Rylander M. Prophylactic intracameral cefuroxime. Efficacy in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg. 2002;28:977–81.
- Group EE study. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the
ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33:978-988. - Jabbarvand M, Hashemian H, Khodaparast M, et al. Endophthalmitis Occurring after Cataract Surgery:
Outcomes of More Than 480 000 Cataract Surgeries, Epidemiologic Features, and Risk Factors. Ophthalmology. 2016;123:295-301. - O’Brien TP, Arshinoff SF, Mah FS. Perspectives on antibiotics for postoperative endophthalmitis prophylaxis: potential role of moxifloxacin. J Cataract Refract Surg. 2007;33:1790-800.
- Yu-Wai-Man P, Morgan SJ, Hildreth AJ, et al. Efficacy of intracameral and subconjunctival cefuroxime in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg. 2008;34:447-451.
- García-Sáenz MC, Arias-Puente A, Rodríguez-Caravaca G, Bañuelos JB. Effectiveness of intracameral cefuroxime in preventing endophthalmitis after cataract surgery: Ten-year comparative study. J Cataract Refract Surg. 2010;36:203-207.
- Friling E, Montan P. Bacteriology and cefuroxime resistance in endophthalmitis following cataract surgery before and after the introduction of prophylactic intracameral cefuroxime: a retrospective single-centre study. J Hosp Infect. 2018;101:88-92.
- Espiritu CRG, Bolinao JG. Prophylactic intracameral levofloxacin in cataract surgery – An evaluation of safety. Clin Ophthalmol. 2017;11:2199-2204.
- Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377-92.
- Vieira IV, Boianovsky C, Saraiva TJ, et al. Safety and efficacy of intracameral moxifloxacin injection for prophylaxis of endophthalmitis after phacoemulsification. Arq Bras Oftalmol. 2017;80:165-167.
- Espiritu CR, Caparas VL, Bolinao JG. Safety of prophylactic intracameral moxifloxacin 0.5% ophthalmic solution in cataract surgery patients. J Cataract Refract Surg. 2007;33:63–68.
- Arbisser LB. Safety of intracameral moxifloxacin for prophylaxis of endophthalmitis after cataract surgery. J Cataract Refract Surg. 2008;34:1114-20.
- Kim SY, Park YH, Lee YC. Comparison of the effect of intracameral moxifloxacin, levofloxacin and cefazolin on rabbit corneal endothelial cells. Clin Exp Ophthalmol. 2008;36:367-70.
- Lane SS, Osher RH, Masket S, Belani S. Evaluation of the safety of prophylactic moxifloxacin in cataract surgery. J Cataract Refract Surg. 2008;34:1451-59.
- Haripriya A, Chang DF, Ravindran RD. Endophthalmitis Reduction with Intracameral Moxifloxacin Prophylaxis: Analysis of 600 000 Surgeries. Ophthalmology. 2017;124:768-775.
- Lucena NP, Pereira IMS, Gaete MIL, et al. Intracameral moxifloxacin after cataract surgery: a prospective study. Arq Bras Oftalmol. 2018;81:92-94.
- Arshinoff SA, Modabber M. Dose and administration of intracameral moxifloxacin for prophylaxis of postoperative endophthalmitis. J Cataract Refract Surg. 2016;42:1730-1741.
- Bowen RC, Zhou AX, Bondalapati S, et al. Comparative analysis of the safety and efficacy of intracameral cefuroxime, moxifloxacin and vancomycin at the end of cataract surgery: a meta-analysis. Br J Ophthalmol. 2018;102:1268-1276.
- Perone JM, Boiche M, Lhuillier et al. Correlation between postoperative central corneal thickness and endothelial damage after cataract surgery by phacoemulsification. Cornea. 2018;0:1-4.
- Bamdad S, Bolkheir A, Sedaghat MR, Motamed M. Changes in corneal thickness and corneal endothelial density after phacoemulsification cataract surgery: a double-blind, randomized trial. Electron Physician. 2018;10:6616-23.
- Wilczynski M, Sepady E, Loba P, et al. Comparison of early corneal endothelial cell loss after coaxial phacoemulsification through 1.8 mm microincision and bimanual phacoemulsification through 1.7 mm microincision. J Cataract Refract Surg. 2009;35:1570-4.
- Nayak BK, Shukla RO. Effect of corneal endothelial cell loss during phacoemulsification: Fortified balanced salt solution versus Ringer lactate. J Cataract Refract Surg. 2012;38:1552-8.
- Ventura ACS, Walti R, Bohnke M. Corneal thickness and endothelial density before and after cataract surgery. Br J Ophthalmol. 2001;85:18-20.
- Chen X, Yu Y, Song X, et al. Clinical outcomes of femtosecond laser assisted cataract surgery versus conventional phacoemulsification surgery for hard nuclear cataracts. J Cataract Refract Surg. 2017;43:486-91.
- Stock G, Ahlers C, Dunavoelgyi R, et al. Evaluation of anterior-segment inflammation and retinal thickness change following cataract surgery. Acta Ophthalmol. 2011;89:369-75.
- Perente I, Utine CA, Ozturker , et al. Evaluation of macular changes after uncomplicated phacoemulsification surgery by optical coherence tomography. Curr Eye Res. 2007;32:241-7.
- Gharbiya M, Cruciani F, Cuozzo G, et al. Macular thickness changes evaluated with spectral domain optical coherence tomography after uncomplicated phacoemulsification. Eye (Lond). 2013:27:605-11.
- Hwang HS, Ahn YJ, Lee HJ, et al. Comparison of macular thickness and inflammatory cytokine levels after microincision versus small incision coaxial cataract surgery. Acta Ophthalmol. 2016;94:e189-94.

