Comparison of Retinal Nerve Fiber Layer Thickness in Elderly Diabetic Patients with and without Peripheral Neuropathy
Bonifacio Buño II, MD, Darby Santiago, MD
Diabetic peripheral neuropathy is one of the most common, long-term complications of diabetes mellitus, affecting up to 50% of all diabetic people. In 2003, it was estimated that elderly American patients comprised 92.5% of the population with diabetic peripheral neuropathy and accounted for 93.1% ($10.2 billion) of the total national health care costs of this condition. Several risk factors are associated with diabetic peripheral neuropathy including smoking, microalbuminuria, dyslipidemia, and retinopathy.1
A serious complication of diabetic peripheral neuropathy is foot ulceration that can lead to amputation. Office-based neurologic examination of the lower extremities can be done to screen for diabetic peripheral neuropathy. It involves a 10-g monofilament test and a modified neuropathy disability score.3 In the presence of peripheral neuropathy, a subject will not feel the monofilament when brushed against his/her skin. The most common types of monofilament used by clinicians are the 4.17, 5.07, and 6.10. Based on experimental data, the 5.07/10-g monofilament is most sensitive in detecting loss of sensation.4 In the study of Dioquino et al. on the utility of monofilament testing in detecting peripheral neuropathy, the sensitivity and specificity rates of the monofilament test in the detection of peripheral neuropathy were 57.1 and 100%, respectively.
Several factors such as age, sex, axial length, and ethnicity can affect retinal nerve fiber layer (RNFL) thickness. Srinivasan et al. reported that RNFL thickness decreases with advancing age. Additionally, RNFL thickness has been found to decrease by 1.27-2.2 μm for every 1 mm increase in axial length. RNFL thickness also varies across different ethnic groups. Asians have thicker RNFL compared to Caucasians and Hispanics. Present data also shows females have thinner RNFL compared to males.7
In diabetic patients, current evidence illustrates that neuronal degeneration can happen before the appearance of microvascular changes. A study by Rodrigues et al. involving 102 patients showed that there is a significant thinning of RNFL in diabetic patients even in the absence of clinically apparent diabetic retinopathy. They concluded that neuroretinal changes occur before vascular signs of diabetic retinopathy.8 These findings were consistent with study conducted by Chhablani et al. in 2015.9 Additionally, Shahidi et al. demonstrated a significant reduction in the inferior RNFL quadrant in relation to the neuropathy disability score. The observed reduction was unrelated to age, retinopathy stage, and glycemic status.
Biological and social factors among different ethnic groups have been implicated in the disparities in prevalence and presentation of diabetes and its complications. Hence, it is important to do this study on Filipinos considering the unique genetics and different sociocultural profile of our population. Additionally, it is also important to explore if certain neuroretinal anatomical changes are related to peripheral neuropathy independent from retinal vascular changes seen in diabetic patients. These neuroretinal changes may cause early unrecognized defects in visual function in diabetic patients with peripheral neuropathy. If significant relationship between RNFL thickness and peripheral neuropathy will be established among Filipinos, then eye care practitioners may become an important part of multidisciplinary teams, including endocrinologists and podiatrists, in treating diabetic complications.
The primary goal of this study is to determine the relationship between diabetic peripheral neuropathy and RNFL thickness among elderly Filipinos with type 2 diabetes mellitus. It also aims to identify the effects of age, sex, retinopathy status, and duration of diabetes on RNFL thickness.
METHODS
Study Participants
This was a cross-sectional study involving 106 patients aged 60 years and older with type 2 diabetes mellitus from the General Medicine and Diabetes Clinics of a tertiary government hospital. All eligible participants have best-corrected visual acuity equal to or better than 6/9, can understand and speak Filipino, and can follow the study procedures. Exclusion criteria include presence of coexisting ocular diseases, intraocular pressure (IOP) of >21 mmHg by Goldmann applanation tonometry, cup- to-disc ratio >0.8 in at least one eye, presence of severe, non-proliferative and proliferative diabetic retinopathy, history of photocoagulation, glaucoma, media opacities that prevent good view of the fundus, and history of neuropathy unrelated to diabetes.
Assessment of Diabetic Peripheral Neuropathy
All participants underwent a 10-gram monofilament test. The test was performed by a neurologist who was masked from the optical coherence tomography (OCT) measurements. With the subject’s eyes closed, the monofilament was first applied on the participant’s upper arm. The subject was instructed to reply in a “yes” or “no” manner when asked if he or she could feel the monofilament. The test was then carried out by applying the monofilament on 4 sites of each foot, particularly the 1st, 3rd, and 5th metatarsal heads, and plantar surface of the distal hallux. Areas of callus were avoided during the examination. Inability to feel the monofilament on at least two sites was recorded as positive for peripheral neuropathy.2,7
Assessment of RNFL Thickness
Each participant underwent measurement of RNFL thickness in both eyes using an OCT. OCT imaging was performed with a Cirrus® HD-OCT (Carl Zeiss Meditec, Dublin, CA, USA) machine centered on the optic nerve head. The Cirrus-1 pt HD-OCT algorithm automatically identifies the center of the optic disc and creates an artificial B-scan in the shape of a circle with a 3.46 mm diameter around it. HD-OCT imaging was generated using the optic disc cube 200 x 200 protocol (software version 6.5.0.772). A global mean RNFL thickness was generated in addition to temporal, superior, nasal, and inferior quadrant averages.
Assessment of Other Variables
The participants were interviewed for their basic demographic information. Diabetic retinopathy was assessed by funduscopic examination carried out through dilated pupils by the primary investigator and were classified according to the Early Treatment Diabetic Retinopathy Study.11
Statistical Analysis
Demographic and clinical data were reported through descriptive statistics using quantitative variables (mean, standard deviation) and qualitative variables (frequency, percentage). Student’s t-test was used to compare normally distributed continuous variables between two groups; while Chi-square test was used to compare categorical variables. A t-test was used to compare global and quadrantal RNFL thickness in those with peripheral neuropathy versus those without peripheral neuropathy. Multivariate regression analysis was done to assess the effect of peripheral neuropathy, retinopathy status, age, sex, and duration of diabetes on global and quadrantal RNFL thickness. Hypothesis testing was done with a 95% level of significance and power of 90%.
RESULTS
One-hundred six (106) subjects were included in the study. There were 31 males (29.2%) and 75 females (70.8%). Mean age [standard deviation] was 65.8 [1.41] years old. Twenty (20 or 18.9%) subjects had peripheral neuropathy while 86 (81.1%) did not have peripheral neuropathy by 10-gram monofilament testing. Comparing the groups with and without peripheral neuropathy, there were significant differences in age (P =0.036) and retinopathy status (P =0.006) (Table 1). Patients with peripheral neuropathy were younger than patients without peripheral neuropathy (63.5 [4.5] vs 67.0 [4.7] years old, respectively). A higher proportion of subjects with peripheral neuropathy had some form of diabetic retinopathy compared to the group without peripheral neuropathy (35% vs 15.1%, respectively). Gender distribution and duration of diabetes did not differ significantly between the 2 groups.
Table 1. Demographic and clinical characteristics of subjects with and without diabetic peripheral neuropathy.
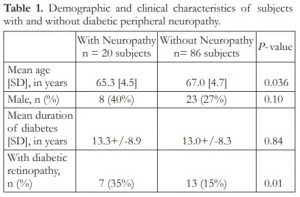
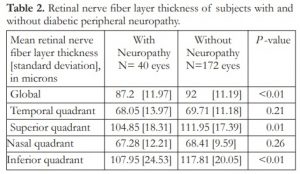
Table 2 shows the RNFL thickness of subjects with and without peripheral neuropathy. Mean global RNFL thickness was 87.2 [12.0] microns in those with peripheral neuropathy compared to 92.0 [11.2] microns in those without peripheral neuropathy. This difference was statistically significant (P <0.01). Significant thinning was also observed in the superior and inferior quadrants in those with peripheral neuropathy compared to the group without peripheral neuropathy. Specifically, mean superior RNFL thickness was 104.9 [18.3] microns in the peripheral neuropathy group compared to 111.9 [17.4] microns in the group without peripheral neuropathy (P =0.01).
While, mean inferior RNFL thickness was 108.0 [24.5] microns in the former compared with 117.8 [20.1] microns in the latter (P <0.01). Lastly, there were no significant differences between the RNFL thickness in the temporal and nasal quadrants between the 2 groups (P =0.21 and P =0.26, respectively).
Table 2. Retinal nerve fiber layer thickness of subjects with and without diabetic peripheral neuropathy.
Multivariate regression analysis revealed that the presence of peripheral neuropathy has a significant effect on the superior (P =0.04), inferior (P =0.01), and global (P =0.02) RNFL thicknesses (Table 3). On the other hand, the presence of peripheral neuropathy has no effect on the temporal (P =0.32) and nasal (P =0.66) RNFL thicknesses. Age, sex, and duration of diabetes do not have an effect on global or quadrantal RNFL thickness. Meanwhile, the presence of diabetic retinopathy was found to be a significant factor to the temporal RNFL thickness (P =0.01) but not for global, inferior, nasal, and superior RNFL thicknesses. A significant thickening in the temporal RNFL quadrant was observed in patients with retinopathy, this finding was inconsistent with the generalized thinning of the global and other RNFL quadrants in the said group of subjects.
Table 3. Multivariate regression analysis of factors associated with global and quadrantal retinal nerve fiber layer thickness.

DISCUSSION
This study compared the RNFL thickness in eyes of subjects with and without diabetic peripheral neuropathy. Results of this study showed that subjects with peripheral neuropathy had significantly reduced global, superior, and inferior RNFL thicknesses compared to subjects without peripheral neuropathy. Thinning in these regions was not influenced by age, duration of diabetes, or presence of diabetic retinopathy. These findings are consistent with papers published by Shahidi et al.10 and Srinivasan7 wherein inferior RNFL thinning was observed in relation to neuropathy by 1.23 and 1.5 microns, respectively.
RNFL thinning in individuals with diabetic peripheral neuropathy was not observed in all quadrants. In this study, there were preferential losses in the superior and inferior RNFL. The superior RNFL is formed by the convergence of the ganglion cell axons from the superior retina, while the inferior RNFL represents axons from the inferior retina. Thinning of the RNFL may indicate retinal damage or loss in the corresponding region. Increased thinning in the superior and inferior RNFL may suggest increased susceptibility of the superior and inferior retinas to initial damage brought about by hyperglycemia compared to the other regions of the retina (macula and nasal retina). In the study by Sugimoto et al., both retinal and RNFL thicknesses in the superior region were found to be significantly thinned out in eyes of diabetic patients in the absence of diabetic retinopathy.13 Tang et al. also demonstrated significantly more microaneurysms and damaged capillaries and pericytes in the superotemporal retinas harvested from diabetic cadavers. Microvascular damage is unequally distributed in the retina even though the hyperglycemia level is the same across all regions. Moreover, Lövestam-Adrian et al. reported reduced electrical potential in the inferior retina of subjects with diabetic neuropathy.15 They suggested diminished electrical potential in a certain region of the retina can be an indicator of neurodegeneration. Additionally, the inferior region of normal human retina has been reported to receive less blood flow per nerve fiber tissue volume compared to the other sectors.16 Due to its relative thickness, the inferior retina requires the greatest amount of oxygen and blood supply. Thus, the high metabolic demand of the inferior RNFL negatively affects its adaptive mechanism to the metabolic stress secondary to diabetes and may make it more prone to ischemic insults.
Statistically, retinopathy has no significant effect on RNFL thickness. Even though manifestation of retinopathy was observed to have a significant effect in the temporal RNFL quadrant, an opposite trend inconsistent with the other quadrants was recorded. The findings of the current study support the hypothesis that neuronal degeneration precedes microvascular abnormalities in diabetes. Rodrigues et al. reported RNFL thinning in diabetic patients without diabetic retinopathy.8 Moreover, reductions in the retinal electrophysiologic parameters have been reported to precede clinical signs of diabetic retinopathy.15 In a postmortem study conducted by Barber, early apoptosis of retinal neural cells was observed in diabetic subjects.14 Shahidi et al. and Srinivasan reported that neuropathy and retinopathy differ in the retinal area of their affectation. The neuropathic effects are more concentrated at the peripapillary nerve fiber layer while retinopathy affects thickness of the parafovea. 7,10
In conclusion, there were significant reductions in the superior, inferior, and global RNFL thickness in subjects with diabetic peripheral neuropathy. These were not influenced by age, sex, duration of diabetes, and presence of diabetic retinopathy. RNFL thinning has an important implication in the management of diabetic patients. This could underlie early and unrecognized defects in visual function in diabetic patients with peripheral neuropathy in the absence of clinical diabetic retinopathy. Future studies are recommended to evaluate the visual function in this group of patients.
REFERENCES
1. Gordois A, Scuffham P, Shearer A, et al. The health care costs of diabetic peripheral neuropathy in the U.S. Diabetes Care. 2003;26:1790-1795.
2. Boulton AJ, Vinik AI, Arezzo JC, Bril, V, et al. Diabetic Neuropathies: A statement by the American Diabetes Association. Diabetes Care. 2005;28:956-962.
3. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, et al. Diabetic Neuropathies: Update on Definitions, Diagnostic Criteria, Estimation of Severity, and Treatments. Diabetes Care. 2010;33:2285-2293.
4. Dros J, Wewerinke A, Bindels PJ, van Weert HC. Accuracy of Monofilament Testing to Diagnose Peripheral Neuropathy: A Systematic Review. Annals of Family Medicine. 2005;7:555–558.
5. Dioquino C, Dellosa M, Reyes J, Panganiban L. Usefulness of Monofilament Testing for Detecting Peripheral Neuropathy I. Acta Medica Filipina. 2009;43:4-9.
6. Adhi M, Aziz S, Muhammad K, Adhi MI. Macular thickness by age and gender in healthy eyes using spectral domain optical coherence tomography. Plos One. 2012;7:e37638.
7. Srinivasan S, Pritchard N, Vagenas D, Edwards K, et al. Retinal Tissue Thickness is Reduced in Diabetic Peripheral Neuropathy. Current Eye Research. 2016;1-8.
8. Rodrigues EB, Urias MG, Penha FM, Badaró E, et al. Diabetes induces changes in neuroretina before retinal vessels: A spectral-domain optical coherence tomography study. Int J Retina Vitreous. 2015;1:4.
9. Chhablani J, Sharma A, Goud A, Peguda HK, et al. Neurodegeneration in Type 2 Diabetes: Evidence From Spectral-Domain Optical Coherence Tomography. Invest Ophthalmol Vis Sci. 2015;56:6333.
10. Shahidi AM, Sampson GP, Pritchard N, Edwards K, et al. Retinal nerve fibre layer thinning associated with diabetic peripheral neuropathy. Diabetic Medicine. 2012;29:e106-11.
11. Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs – an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98:786-806
12. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report no 1. Arch Ophthalmol. 1985;103:1796-1806
13. Sugimoto M, Sasoh M, Ido M, Wakitani Y, et al. Detection of early diabetic change with optical coherence tomography in type 2 diabetes mellitus patients without retinopathy. Ophthalmologica. 2005;219:379–385.
14. Barber AJ, Lieth E, Khin SA, Antonetti DA, et al. Neural apoptosis in the retina during experimental and human diabetes: early onset and effect of insulin. J Clin Invest. 1994;102:783–791.
15. Lövestam-Adrian M, Gränse L, Andersson G, Andreasson S. Multifocal visual evoked potentials (MFVEP) in diabetic patients with and without polyneuropathy. The Open Ophthalmol J. 2012;6:98-103.
16. Harris A, Ishii Y, Chung HS, Jonescu-Cuypers CP, et al. Blood flow per unit retinal nerve fibre tissue volume is lower in the human inferior retina. Br J Ophthalmol. 2003;87:184–188.

