A comparative study between the Schwind and Zyoptix XP microkeratomes
Maria Cecilia P. Garcia, MD, Joanne A. Barleta, MD, Gladness HA Martinez, MD, Robert Edward T. Ang, MD
LASER in situ keratomileusis (LASIK) is a widely accepted procedure in managing refractive errors. However, laser correction is limited by the patient’s corneal thickness. The need to deliver correction for higher errors
of refraction without compromising corneal strength has paved the way for newer-generation microkeratomes and other technologies that create thinner flaps making more corneal tissue available for correction.1
There are different microkeratomes in use today. The main differences lie in the type of movement (straight, pivoting, or pendular), applanation of the cornea during cut(convex or planar), and position of the hinge (superior or nasal). The Schwind Carriazo-Pendular (CP) microkeratome is pendular with convex applanation. It produces a superior hinge. This convex applanation provides a nearly constant pressure on the cornea during the cutting process, producing almost uniform thickness throughout the flap area. The constant pressure applied by the CP is presumed to lower the risk of buttonhole compared with planar applanation microkeratomes in which the central pressure is higher than the peripheral.2 The Zyoptix XP (XP) is a pivot microkeratome with planar applanation. It produces a meniscus-formed flap, which is thinner in the center and thicker in the periphery. The keratome cuts in a constant plane, which produces a smoother corneal bed, thus also suggesting less chance of a buttonhole.3-4 Evaluating flap thickness and quality created by different keratomes is important. With an instrument that offers greater predictability in flap thickness, the surgeon
can reasonably estimate residual bed thickness and avoid ectasia.5 The shearing force due to differences in keratome movement and bed contour may affect visual results and induce unwanted aberrations.6-7 Flap complications such as buttonholes, displacement, and striae may occur with overly thin, friable flaps.8
This study compared the differences in outcomes (flapthickness reproducibility, vision, contrast sensitivity, and
higher-order aberrations) of LASIK between Schwind CP microkeratome and Zyoptix XP microkeratome.
METHODOLOGY
This is a prospective, randomized, subject-masked, comparative clinical study of 32 eyes of 16 patients seen
in a single center from July 2008 to February 2009. The research protocol followed the guidelines of the
Helsinki Declaration and was approved by the hospital’s ethics review board. All patients were fully informed of
the nature and details of the procedure. The scope of the study, including all the risks and benefits involved, were explained. Informed consent was obtained from all To be included, patients must:
• be at least 18 years old;
• have myopia with or without astigmatism in both eyes, but with stable refraction;
• have stopped using soft contact lenses for at least 1 week or hard contact lens for at least 3 weeks;
• have estimated residual corneal-bed thickness of 280 µm and above;
• be willing to undergo LASIK in both eyes. Excluded were patients who:
• have preexisting systemic diseases that could hinder wound healing;
• are pregnant or lactating;
• have active ocular inflammation and infection;
• have a history of herpes simplex or herpes zoster keratitis;
• have signs of corneal pathologies, corneal warpage on topography;
• have unstable refraction;
• have had previous eye surgeries or eye trauma;
• have cataract, glaucoma, or other optic-nerve diseases;
• have retina pathologies;
• will undergo PRK or LASEK.
All patients underwent refractive-screening protocol which included history taking, visual-acuity measurements, manifest and cycloplegic refractions, dim-light pupil-size determination, slitlamp examination, intraocular pressure check, ultrasonic pachymetry, Schirmer’s testing, corneal topography using Orbscan IIz, Zywave undilated and dilated wavefront aberrometry measurements, and dilated-fundus examination.
All laser treatments for each eye were targeted for emmetropia using the personalized-treatment software
planner (Bausch & Lomb, Rochester, NY, USA). The surgeries were performed by a single surgeon. The right
eye was treated first. A randomization table was used to determine which eye would undergo flap creation using either CP microkeratome or XP microkeratome. The patients were blinded as to the type of microkeratome. On the operative eye, asepsis and antisepsis technique with 10% betadine was applied around the lid and periorbital adnexa, followed by sterile draping of the eye and placement of the lid speculum. Pachymetry was done on the central cornea before placement of keratome. Flaps were created using either the CP or the XP microkeratome based on the randomization table. The flaps were lifted and pachymetry measurements were done on the dry stromal bed. The pachymeter automatically took 25 readings and the mean reading was recorded. To obtain the flap thickness per eye, the stromal-bed-thickness measurement was subtracted from the corneal thickness prior to flap cutting. Laser ablation was performed using the Technolas 217z and the flap repositioned by floating and then dried.
Postoperative medications were gatifloxacin( Zymar, Allergan, Irvine, CA, USA ) and 1% prednisolone acetate
(Pred Forte, Allergan, Irvine, CA, USA) 4x a day for at least 2 weeks. Examinations were scheduled at 1 day, 7 days, 1 month, 3 months, and 6 months postoperatively. Uncorrected visual acuity (UCVA) and best-corrected visual acuity (BCVA) were measured at each postoperative visit. Contrast-sensitivity tests, topography, and aberrometry were performed at 1, 3, and 6 months follow-up. Statistical data were collected and analyzed using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) data-analysis tool pack. Two-tailed paired student ttest was performed with a significance level of p < 0.05. Means and standard deviations were also obtained. Visual acuity (VA) was expressed in the logarithm of minimum angle of resolution (logMAR) scale using a VA conversion chart.
RESULTS
Thirty-two eyes of 16 patients were included in the study. Six were male and 13 female, with a mean age of 34 ± 9.3 years (range, 21 to 48 ). The preoperative UCVA, spherical equivalent, and central pachymetry were similar in both groups (Table 1). Flap thickness and complications The mean flap thickness created was 97 ± 13 µm (range, 81 to 116 µ) using the CP and 146 ± 27 µm (range, 71 to 181 µm) using the XP. The mean deviation from the predicted thickness was –13 ± 13 µm and 26 ± 27 µm for the CP and XP respectively. The difference between the groups was statistically significant (p = 0.002). In the CP group, 3 eyes had flap displacement, 1 had loose epithelium and 1 had flap striae. There were no complications in the XP group. No evidence of ectasia was noted in all eyes. Visual results At 6 months postoperatively, UCVA was 20/20 in 93% and 86% of eyes in the CP and XP groups respectively. Mean UCVA was 20/20 (range, 20/15 to 20/20) in the CP and 20/25 (range, 20/15 to 20/30) in the XP groups. UCVA was 20/30 or better in all eyes in the 2 groups (Figure 1). The BCVA was 20/20 in 100% and 93% of eyes in the CP and XP groups respectively. Mean BCVA was 20/20 in both groups (range, 20/15 to 20/20). All eyes achieved BCVA of 20/25 (Figure 2). There was no statistical difference in the UCVA and BCVA in either group (p = 0.27 for CP and p = 0.16 for XP). Preoperatively, the mean spherical equivalent was –4.50D and –4.70 in the CP and XP. The mean sphere was –4.00D in both groups. The mean cylinder was –0.9 and –0.8
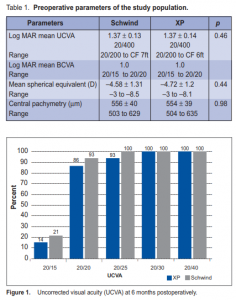

respectively (Table 2). At 6 months postoperatively, the mean spherical equivalent was –0.04D in the CP and –0.1 in the XP groups. The mean sphere was –0.02D and 0.03 and the mean cylinder was –0.1 and –0.27 respectively (Table 2). At 6 months, contrast sensitivity both at photopic and mesopic conditions, with and without glare, were all measured at 1.5, 3, 6, 12, and 18 cycles/degree (cpd) (Figures 3 and 4). Contrast sensitivity was better in the
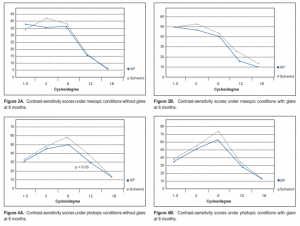
CP than in the XP group, but most measurements did not reach statistical significance except those under
photopic conditions without glare at 12 cpd spatial frequency (p = 0.03). At 6 months, 21% of eyes in the CP group gained 1 line compared with 14% in the XP group. Seven percent in the XP group lost 1 line compared with none in the CP group. Higher-order aberrations Preoperatively, mean spherical aberration was –0.12 µm in the CP and–0.11 µm in the XP groups. Mean total higher-order aberration was 1.56 and 1.30 respectively.
The difference in the aberrations between the 2 groups was not statistically significant (Table 3). At 6 months postoperatively, there was a smaller mean change of spherical aberration in the CP compared with the XP (Table 3), and the difference was statistically significant (p = 0.03). The total higher-order aberrations
were similar in both groups. Mean change in the trefoil, coma, and quadrafoil were also comparable between the groups.
DISCUSSION
This prospective study compared contralateral eyes of the same patient that were randomized to receive flaps
created either by a CP or an XP microkeratome during a wavefront-guided LASIK treatment for myopia or astigmatic myopia. Our data showed that the mean flap thickness (97 ± 13 µm) created by the Schwind
microkeratome was thinner than the labeled predicted thickness (110 µm). In contrast, the mean flap thickness (146 ± 27 µm) created with the XP microkeratome was thicker than predicted (120 µm). These differences were
statistically significant. Thinner flaps have the advantage of preserving more untreated cornea; hence, the biomechanical integrity is less altered.5, 9 Thicker flaps may inadvertently increase risks of ectasia if the residual stroma is less than what the surgeon estimated it would be. On the other hand, studies also reported that thinner flaps were more difficult to manage and had greater tendency to develop flap striae, displacement, and irregular astigmatism.8, 10 The results of this study are consistent with these reports since only the thinner flaps in the CP group had flap problems. Despite the complications encountered, they did not affect visual outcomes in the CP group. The difference in flap thickness did not seem to affect visual outcome. There were no statistical differences in the UCVA, BCVA, and contrast sensitivity in both groups. In this study, the flap thickness exhibited an impact on the postoperative spherical aberration. Six months postoperatively, eyes with flaps created using the Schwind microkeratome had smaller mean change in spherical aberration. Spherical aberration, which is symmetrical to the visual axis of the eye, has been found to be substantially
increased after LASIK.7, 11-12 It is hypothesized that flap thickness bears impact on the spherical aberration.
Suggested sources of induced spherical aberration after LASIK included biomechanical responses and epithelialthickness modulation during the wound-healing process. In our study, it was difficult to determine the exact cause of the difference in spherical aberration. Our data seemed to suggest that the thinner flaps created by the CP may be the only identifiable difference and, therefore, the most likely contributing factor.
In summary, CP-created flaps were thinner and showed more predictability than the XP, but they resulted in more complications. Total higher-order aberration was similar in both groups, but spherical aberration was increased in the XP group. Despite these differences, the vision and refractive results were similar for both groups. We conclude that flap quality and thickness may affect flap handling and lead to complications, but have little or no effect on visual outcome. We recommend that future studies should involve larger sample size and comparison of other mechanical microkeratomes or femtosecond lasers to determine if flap quality and thickness do play a role in visual outcome.
References
1. Durairaj VD, Balentine J, Kouyoumdjian G, et al. The predictability of corneal-flap thickness and tissue laser ablation in laser in situ keratomileusis. Ophthalmology 2000; 107: 2140-2143.
2. Arbelaez MC. The Schwind Carriazo-Pendular microkeratome. Tech Ophthalmol 2009; 7: 11-14.
3. Malonez R. The Zyoptix XP: an advance in mechanical microkeratome technology. Refractive Eye Care 2005; 9: 10-13.
4. Stonecipher K, Ignacio T, Stonecipher SB. Advances in refractive surgery: microkeratome and femtosecond laser-flap creation in relation to safety, efficacy, predictability, and biomechanical stability. Curr Opin Ophthalmol 2006; 17: 368- 372.
5. Thomas HO, Arthur CK, Chen MCRS, et al. Comparison of corneal-flap thickness between primary eyes and fellow eyes using Zyoptix XP microkeratome. J Cat Refract Surg 2007;12: 2049-2053.
6. Krueger RR, Dupps WJ Jr. Biomechanical effects of femtosecond and microkeratome-based flap creation: prospective contralateral examination of two patients. J Refract Surg 2007; 23: 800-807.
7. Cheng ZY, He JC, Zhou XT, Chu R Y. Effect of flap thickness on higher-order wavefront aberrations induced by LASIK: a bilateral study. J Refract Surg 2008; 24: 524-529.
8. Kymionis G, Portaliou D, Tsiklis N, et al. Thin LASIK flap creation using the Schwind Carriazo-Pendular microkeratome. J Refract Surg 2009; 25: 33-36.
9. Pallikaris GI, Katsanevaki VJ, Panagopoulou SI, et al. Laser in situ keratomileusis intraoperative complications using one type of microkeratome. Am J Ophthalmol 2002; 109: 57-63.
10. Waheed S, Chalita MR, Xu M, Krueger RR. Flap-induced and laser-induced ocular aberrations in a two-step LASIK procedure. J Refract Surg 2005; 21: 346-352.
11. Miller JM, Anwaruddin R, Straub J, Schwiegerling J. Higher-order aberrations in normal, dilated intraocular lens, and laser in situ keratomileusis corneas. J Refract Surg 2002; 18: S579-S583.
12. Mrochen M, Kaemmerer M, Mierdel P, et al. Increased higher-order aberrations after laser refractive surgery: a problem of subclinical decentration. J Cataract Refract Surg 2001; 27: 362-369

