Relationship of Diabetic Retinopathy with Ankle Brachial Index and Microalbuminuria in Type 2 Diabetics
Emerson Jay B. Molina, MD, Ronald A. Yutangco, MD, Maria Angela S. Cruz-Anacleto, MD, Jennifer Doria-Del Castillo, MD, Patricia J. Aguinod-Cheng, MD
Diabetic retinopathy (DR) has been associated with other microvascular and macrovascular complications of diabetes mellitus. The concordance of microvascular abnormalities, such as diabetic retinopathy and microalbuminuria, has been well reported in type 1 diabetics.1,2 However, the association of microalbuminuria and diabetic retinopathy in type 2 diabetics is controversial and current studies provided
contradicting results.1,3,4,5 Studies have reported the association of diabetic retinopathy with markers of cardiovascular disease.6-8 Among these markers is the ankle/brachial index (ABI), a simple non-invasive method of determining the presence of peripheral arterial disease (PAD). The relationship of ABI scores and PAD with diabetic retinopathy has been well studied but the results are varied.6,7,9,10
In this study, we investigated the relationship of diabetic retinopathy with ankle/brachial index
scores and the presence of microalbuminuria. We also determined the association of the severity of
diabetic retinopathy with abnormal ABI scores and the presence of microalbuminuria.
METHODOLOGY
Study Population
Patients with type 2 diabetes, aged 40-85 years, were recruited from the outpatient department of a
tertiary hospital Participation was voluntary and an informed consent was obtained. Those with dense media opacity obscuring the view of the posterior pole, glaucoma, age-related macular degeneration,
high myopia were excluded. Patients who have other retinal diseases or had undergone ocular procedures
that made classifying the diabetic retinopathy difficult were also excluded.
Determination of Ankle/Brachial Index (ABI)
Ankle/brachial index was determined by measuring the resting systolic blood pressure with the patient in a supine position using a mercurial sphygmomanometer and a handheld doppler device (Otsuka Medical, Inc.). The ABI was computed using the lowest systolic pressure of either the dorsalis pedis artery or the posterior tibialis and the highest brachial systolic pressure. A cut-off value of <0.9 and >1.3 was used to define low and high ABI, respectively.11
Determination of Microalbuminuria
Early morning urine aliquots were obtained from the patients. The dipstick was dipped in urine for 5
seconds, leaving the buffer coating just above the surface of fluid, and the reading was performed exactly 5 minutes after dipping.12 Discrete readings of 0, 10, 20, 50, and 100 mg/ml provided a semiquantitative estimation of albumin concentration.
Assessment for Diabetic Retinopathy
Ophthalmic examination was performed within 2 weeks after ABI and microalbuminuria determination. Patients underwent visual acuity and anterior segment evaluation. Dilated fundus examination was performed using contact lens biomicroscopy to assess the presence and severity of diabetic retinopathy. Indirect ophthalmoscopy was done to detect any peripheral lesions. Findings were verified by a retina specialist. The International Grading System for
Diabetic Retinopathy was used for staging.13 Patients with mild and moderate nonproliferative diabetic retinopathy (NPDR) were grouped under mild diabetic retinopathy and those with severe NPDR and
proliferative diabetic retinopathy were grouped under severe diabetic retinopathy. The results of the ABI and microalbuminuria were initially withheld from the ophthalmic examiners until all qualified patients had been assessed for the presence and stage of diabetic retinopathy.
Statistical Analysis
Data were provided as the mean +/- standard deviation for continuous variables or percentage for
categorical variables. Baseline characteristics between the two groups were compared using the z-test.
For testing the association of diabetic retinopathy with ABI and microalbuminuria, the binary logistic
regression was used. A level of p<0.05 was considered statistically significant. Analyses were performed using
SPSS version 17.
RESULTS
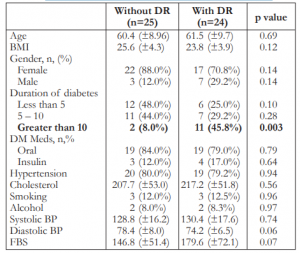
The presence of diabetic retinopathy was confirmed in 24 of the 49 patients (49%) who were qualified for the study. Of these, 11 (45.8%) were in the mild group and 13 (54.1%) severe group. The general characteristics of the study groups with and without retinopathy are summarized in Table 1, showing no significant differences between the 2 groups except for duration of diabetes of more than 10 years.
Table 1. Characteristics of the study population (N=49).
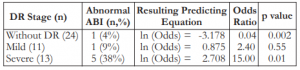
Our data showed that patients with no diabetic retinopathy have lesser probability of having an abnormal ABI score (p = 0.002). The odds ratio of 0.04 meant that patients diagnosed with diabetic retinopathy were (inverted odds) 23.8 times more likely to have an abnormal ABI score than those who have no diabetic retinopathy. On the other hand, severe diabetic retinopathy has a significant relationship with ABI score (p = 0.01), where patients were 15.0 times more likely to have an abnormal ABI score than those who have milder forms of diabetic retinopathy. The presence of mild diabetic retinopathy has a smaller effect (p = 0.55) on the occurrence of an abnormal ABI score, where the likelihood was 2.4 times as compared to the no diabetic retinopathy group (Table 2).
Table 2. Association of diabetic retinopathy with ankle/brachial index scores.
Patients with no diabetic retinopathy have lesser probability of having microalbuminuria (p = 0.001). The odds ratio of 0.09 meant that patients diagnosed with diabetic retinopathy were (inverted odds) 10.99 times more likely to have microalbuminuria than those who have no diabetic retinopathy. Mild diabetic retinopathy was not significantly related to the presence of microalbuminuria (p = 0.99). The presence of severe diabetic
retinopathy, however, was significantly associated with microalbuminuria (p = 0.01), where patients were 13.75 times more likely to have microalbuminuria than those who were diagnosed with milder stages
of diabetic retinopathy (Table 3).
Table 3. Association of diabetic retinopathy with micro- albuminuria.

DISCUSSION
The findings in our study correlated with the results of large population-based studies in the
literature3,6 where positive associations were reported between diabetic retinopathy and the presence of ABI
abnormalities and microalbuminuria. However, there were few studies reported on these complications using the same patient population.14,15 In the assessment of diabetic retinopathy, we employed contact lens biomicroscopy for optical quality and greater axial magnification. This methodology differed from those in the reported literature where fundus photography was commonly used for evaluation.5-8 Logistic reasons aside, we believed that it reflected the primary method of diagnosing diabetic retinopathy in the local clinical setting. The interval between determination of ABI and microalbuminuria and that of the ophthalmic examination in our study was less than a month. We did not find any literature describing the optimal time between the examinations. However, comparing the study of Kawasaki et. al6 where intervals reached up to 2 years in some cases, ours was significantly shorter. With this modification, we believed that we have minimized the chances of including subjects whose retinopathy status was worse on ophthalmic exam than when the ABI and microalbuminuria were determined. A low ABI score (<0.9) has classically been used
as a marker for PAD and as a predictor of increased morbidity and mortality.6,7,9 A critical review of ABI by Khan and colleagues showed up to four-fold increase in cardiovascular disease and mortality in patients with abnormal ABI.11 We used the low ankle pressure method for determining ABI (LAP ABI) wherein sensitivity and specificity were reported as 89 and 93%, respectively. A high ABI score (>1.3) was considered
abnormal but not indicative of PAD. Rather, it denoted vessel incompressibility.11 The Strong Heart Study showed that low and high ABI scores were equally predictive of cardiovascular disease & mortality among patients.16 Hence, in our analyses a low or high ABI score was reported as an abnormal finding. Our findings showed that an advanced stage of diabetic retinopathy was associated with an increased likelihood of having an abnormal ABI score. Microalbuminuria is defined as urinary excretion of 30-300 mg/day. It is believed that its presence does not indicate presence of a kidney disease per se, but rather a secondary process affecting kidney physiology.17,18 The urine micral test provided a semiquantitative estimation of albumin excretion
with a reported sensitivity of 87-89% and specificity of 88-98% with overall accuracy of 88-92%12 in
detecting microalbuminuria. We found a significant association of advanced diabetic retinopathy with the
presence of microalbuminuria. This may be explained by the view that microalbuminuria might represent a
generalized vascular endothelial dysfunction.3,17,18 We found no significant association between milder stages of diabetic retinopathy and microalbuminuria. Limitations of this study should be noted. We have a relatively small sample size and as a crosssectional study, it did not study the cause-and-effect relationship among diabetic retinopathy, albuminuria and ABI scores. Moreover, our diabetic retinopathy grading did not employ stereoscopic fundus photography which is considered the gold standard. The strengths of this study were the use of a population of purely type-2 diabetics, the short interval between determination of ABI and microalbuminuria and assessment of retinopathy, and the ability to study the association of diabetic retinopathy with ABI and microalbuminuria in the same population. In conclusion, our study showed that patients with severe diabetic retinopathy were more likely to have abnormal ankle/brachial index scores and microalbuminuria. Thus, those presenting with severe diabetic retinopathy were not only at risk of losing their sight but also at higher risk of developing lifethreatening systemic vascular and renal complications of diabetes.
REFERENCES
1. The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial (DCCT). Arch Ophthalmol 1995;113:36–51.
2. Lovestam-Adrian M, Agardh E, Agardh CD. The temporal development of retinopathy and nephropathy in type 1 diabetes mellitus during 15 years diabetes duration. Diabetes Res Clin Pract 1999;45:15-23.
3. Rani P, Raman R, Gupta A, et al. Albuminuria and diabetic retinopathy in type 2 diabetes mellitus. Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN-DREAMS, report 12). Diabetology and Metabolic Syndrome 2011; 3:9.
4. Potisat S, Srisubat A, Krairttichai A, Jongsareejit A. The relationship between microalbuminuria by using urine dipsticks and diabetic retinopathy in type 2 diabetes mellitus. J Med Assoc Thai 2008; 6:846-51.
5. Manaviat M, Afkhami M, Shoja M. Retinopathy and albuminuria in type 2 diabetic patients. Int J Endocrinol Metab 2005; 4:153-157.
6. Kawasaki R, Cheung N, Islam A, et al. Is diabetic retinopathy related to subclinical cardiovascular disease? Ophthalmology 2011; 118:860-865.
7. Yun YW, Shin MH, Lee YH, et al. Arterial stiffness is associated with diabetic retinopathy in Korean type 2 diabetic patients. J Prev Med Public Health 2011; 44:260-266.
8. Tanaka K, Kawai T, Saisho Y, et al. Relationship between stage of diabetic retinopathy and pulse wave velocity in Japanese patients with type 2 diabetes. J Diabetes Res 2013; 2013:193514.
9. Ricciardi G, Vaccaro O, Rivellese A, et al. Association between diabetic retinopathy and impaired peripheral arterial circulation in insulin-dependent diabetic patients. Arterioscler Thromb Vasc Biol 1988; 8:509-514.
10. Tryniszewski W, Gadzicki M, Maziarz Z, et al. Progression of diabetic retinopathy correlated with muscle perfusion disturbances of the lower limbs, with clinically important diagnostic recommendations. Arch Med Sci 2010;6:904-911.
11. Khan T, Farooqi F, Niazi K. Critical review of the ankle brachial index. Curr Cardiol Rev 2008;4:101-106.
12. Minetti E, Cozzi M, Granata S, Guidi E. Accuracy of the urinary albumin titrator stick ‘Micral-Test’ in kidney-disease patients. Nephrol Dial Transplant 1997;12:78-80.
13. Wilkinson C, Ferris F, Klein R, et al. The Global Diabetic Retinopathy Project Group. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology 2003;110:1677–1682.
14. Papanas N, Symeonidis G, Mavridis G, et al. Ankle-brachial index: a surrogate marker of microvascular complications in type 2 diabetes mellitus? Int Angiol 2007;26:253-7.
15. Chandy A, Pawar B, John M, Isaac R. Association between diabetic nephropathy and other diabetic microvascular and macrovascular complications. Saudi J Kidney Dis Transplant 2008;19:924-928.
16. Resnick H, Lindsay R, McDermott M, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality (The Strong Heart Study). Circulation 2004;109:733-739.
17. Sobngwi E, Mbanya JC, Moukouri EN, Ngu KB. Microalbuminuria and retinopathy in a diabetic population of Cameroon. Diabetes Res Clin Pract 1999;44:191-196.
18. Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep 2010;12:364- 368.

