Effect of topical ketorolac tromethamine and topical nepafenac on maintaining pupillary dilation during phacoemulsification
Richard Atanis, MD, Prospero Ma. Tuaño, MD, Jay Vicencio, MD, Jose Ma. Martinez, MD, Lee Verzosa, MD
PHACOEMULSIFICATION with intraocular-lens (IOL) implantation is the current surgical treatment of choice for cataract extraction.1-3 To prevent complications during surgery, there should be adequate pupillary dilation for better visualization of the posterior chamber. Evidence has shown that intraocular manipulation can trigger the inflammatory cascade, releasing cyclooxygenase (COX) and prostaglandins within the eye causing miosis. During cataract surgery, maintenance of mydriasis is necessary to facilitate proper incision of the anterior capsule, safe removal of the cataract, and implantation of intraocular lens. Mydriatics and antiprostaglandins are routinely applied preoperatively to facilitate cataract extraction and prevent intraoperative miosis.4 Previous studies have demonstrated the effectiveness of various topical nonsteroidal antiinflammatory drugs (NSAIDs) (indomethacin, flurbriprofen, suprofen) in preventing miosis during cataract surgery compared to placebo.5 Newer topical NSAIDs also showed similar favorable effects. Coste6 showed that nepafenac given 3 times a day 1 day before cataract surgery was superior to tobramycindexamethasone eye drops in maintaining intraoperative mydriasis measured at 4 different stages of the surgery. Solomon5 compared the effects of topical 0.5% ketorolac tromethamine ophthalmic solution with topical 0.03% flurbiprofen sodium on the inhibition of surgically induced miosis during phacoemulsification. Ketorolac provided a more stable mydriatic effect throughout the surgical procedure. This study compared the effect of 2 newer topical NSAIDs widely available in the Philippines—ketorolac 0.5% and nepafenac 0.1%. Specifically, this study determined the horizontal and vertical pupillary diameters in 4 different stages of phacoemulsification; compared pupillary diameter measurements among the ketorolac, nepafenac, and placebo groups; and determined the total loss and percent total loss of mydriasis.
METHODOLOGY
We conducted a prospective, randomized, doublemasked comparative study involving 47 eyes of 44 Filipino patients diagnosed with mature cataract who underwent cataract surgery by phacoemulsification and capsular bag IOL implantation in a tertiary hospital from March to August 2010. Included were patients who:
• were 40 years of age or older,
• had been diagnosed with mature cataract according to the Lens Opacities Classification System (LOCS III), with classification NO and/or NC 2–3,
• were scheduled for cataract surgery by phacoemulsification and capsular bag IOL implantation,
• had normal funduscopic exam (if retina view was possible),
• had history of unremarkable phacoemulsification with capsular-bag IOL implantation to the contralateral eye, and
• had continuous, circular capsulorhexis of 5 to 6 mm diameter.
The exclusion criteria included:
• history of ocular inflammatory or infectious eye disease,
• treatment of eye infection within 30 days prior to inclusion in the study,
• alterations of the ocular surface (e.g., dry eye),
• history of ipsilateral ocular surgery and/or trauma,
• history of any neuro-ophthalmologic pathologies,
• knowledge or suspicion of allergy or hypersensitivity to the preservatives, steroids, topical NSAIDs, or any other component of the study medication,
• use of topical ophthalmic medications,
• use of topical or systemic steroids within 30 days prior to inclusion in the study,
• use of topical or systemic NSAIDs within 14 days prior to inclusion in the study,
• diagnosis of diabetes mellitus with/without diabetic retinopathy and/or macular edema,
• preoperative mydriasis less than 6 mm prior to the study,
• “Phaco time” of >1.5 minutes,
• intraoperative posterior capsular rent with or without vitreous loss,
• use of intraoperative intracameral epinephrine, • ocular alteration preventing adequate mydriasis (eg. synechiae, iris atrophy),
• use of contact lens at anytime before the surgery,
• surgical events that may hasten pupillary constriction (eg. inadvertent manipulation/aspiration of the iris, incarceration of iris into the main wound secondary to an accidentally shortened or mis-angled main corneal tunnel),
• use of tamsulosin or other analogous systemic medications that may induce increased tendency for miosis intra-operatively (intra-operative floppy iris syndrome or IFIS).
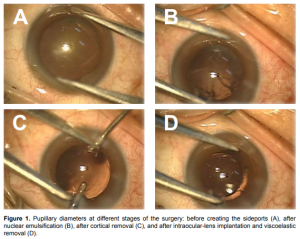
Preoperatively, all subjects underwent a thorough ophthalmic examination. Past medical and surgical history, and use of concurrent medications were extensively reviewed. Best-corrected visual acuity (BCVA) using the ETDRS chart, slit-lamp biomicroscopy, intraocular pressure by Goldmann applanation tonometry, and dilatedfundus examination were done. A general surgical consent form was obtained from all patients. Patients who underwent phacoemulsification were eligible for inclusion. They were randomly assigned to each of the 3 groups based on which of the 3 sealed envelopes was chosen by a junior resident at the time of surgery. Patients received 1 drop of the assigned topical NSAID or balanced salt solution (BSS) (control group) every 15 minutes for 4 doses (~20ml) to the operative site one hour prior to the scheduled operation. Five minutes later, tropicamide 0.5% with phenylephrine 0.5%, 1 drop every 15 minutes for 4 doses was instilled in all treatment groups. The surgeons and the patients were unaware of the type of test drops given. All subjects underwent cataract surgery by phacoemulsification using the Millenium machine (Microsurgical System, Houston, TX, USA). A one-piece, monofocal, foldable acrylic IOL implantation inside the capsular bag under topical anesthesia (proparacaine) was done by 4 surgeons (one consultant and three senior residents). The surgeons used the same operative technique on all patients as follows. Two 1-mm side-ports, a 2.75-mm temporal clear corneal incision, and a 5- to 6-mm continuous curvilinear capsulorhexis were made. Phacoemulsification parameters were established prior to all surgeries and were the same in all patients. Balanced salt solution without epinephrine was used for corneal irrigation. The corneal incisions were left unsutured at the close of surgery. To ensure the standardization of illumination during pupillary measurement, all surgeons used the same microscope (Carl Zeiss OPMI VISU 210 S88) and the illumination was kept constant (0.5 to 0.7) in all cases. The principal author measured the horizontal and vertical pupillary diameters. A sterile caliper was placed over the cornea and measurements were taken, in millimeters, under the microscope at the following stages of surgery: 1) before creating side ports, 2) after nuclear emulsification, 3) following cortex aspiration, and 4) after implantation of an acrylic foldable IOL with viscoelastic removal (Figure 1A-D). The preset standard magnification (0,75x) of the operating microscope was ensured at each of the 4 time points. The primary outcome measures were the mean horizontal and vertical diameters of the pupil during the four different stages of phacoemulsification. Other data collected were age, gender, laterality of the eye operated on, and the corresponding category to which they were assigned. Frequency, percentage, mean and standard deviation were used to describe demographic characteristics and values of pupillary measurements. Comparisons of categorical variables were analyzed using chi square or Fisher exact tests, where applicable. Analysis of variance (ANOVA) was used to determine differences between groups at each stage of surgery, as well as changes from baseline. All analyses were two-tailed, with p<0.05 considered as significant. Analyses were performed using Statistical Package for Social Sciences (SPSS) for Windows, version 16.0.
Figure 1. Pupillary diameters at different stages of the surgery: before creating the sideports (A), after nuclear emulsification (B), after cortical removal (C), and after intraocular-lens implantation and viscoelastic removal (D).
RESULTS
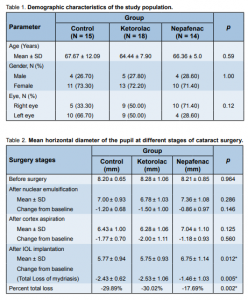
A total of 47 eyes of 44 patients, 13 males and 34 females, were included in the study. The mean age was 66.04 ± 8.87 years. There was no significant difference in age, gender, and laterality of eye operated on among the three groups (Table 1). Significant differences among the three groups were seen after IOL implantation, with the nepafenac group having the largest mean diameters in both horizontal (p = 0.012) and vertical (p = 0.012) pupil measurements (Tables 2 and 3). Comparison of total loss of mydriasis, which is the difference between pupil diameter before surgery and after IOL implantation, revealed significant differences in both horizontal (p = 0.005) and vertical (p = 0.009) pupil measurements with the nepafenac group having the least change from baseline. The least loss in mydriasis in horizontal pupil diameter was observed in the nepafenac group with 17.69% loss which was significantly lower (p = 0.002) compared to 29.89% and 30.02% losses of the control and ketorolac groups respectively (Table 2). Similarly, the vertical pupil measurements showed significant differences in percent of total loss with the nepafenac group having 17.32% compared with the 27.89% and 28.80% total losses of the mydriasis of the ketorolac and control groups respectively (Table 3).
Table 1. Demographic characteristics of the study population.
Table 2. Mean horizontal diameter of the pupil at different stages of cataract surgery.
Table 3. Mean vertical diameter of the pupil at different stages of cataract surgery.

DISCUSSION
Mechanical ocular trauma from phacoemulsification can cause various ocular changes, such as conjunctival hyperemia, inflammation, pain, cystoid macular edema, breakdown of the blood–aqueous barrier, rise in intraocular pressure, and most especially surgically-induced miosis creating access for cataract removal difficult.7-8 Prostaglandins play an important role in these changes. NSAIDs inhibit COX enzymes that promote prostaglandin production; hence, providing both analgesic and antiinflammatory activities.6 Ophthalmic NSAIDs are used to decrease the various changes brought about by intraocular surgeries. Due to the topical nature of this drug class, systemic absorption is minimal. Nepafenac 0.1%, after topical dosing, is subsequently converted by ocular tissue hydrolases to amfenac, which is thought to inhibit the action of the cyclooxygenase prostaglandin H synthase.9 Nepafenac 0.1% met its primary objective in this present study by showing advantage over the control group in terms of maintaining mydriasis during phacoemulsification. In addition, nepafenac 0.1% has also shown to be more effective than placebo at maintaining mydriasis at every stage of the surgery. Most interesting, however, is the comparison between nepafenac 0.1% and ketorolac 0.5%. Previous studies have established the effectiveness of ketorolac 0.5% for the treatment of both pain and inflammation following cataract surgery.10 Consequently, ketorolac 0.5% was used as a standard against which the efficacy of nepafenac 0.1% was measured. In this study, nepafenac 0.1% reached statistical superiority compared to ketorolac 0.5% in all four stages of phacoemulsification. Nepafenac has been shown to penetrate the cornea rapidly and provides a complete and longer-lasting inhibition of prostaglandin synthesis and vascular permeability.11-12 Perhaps, this advantage in absorption and bioavailability was the reason behind its superiority in maintenance of mydriasis seen in this study. Prescribing a consistent technique as well as dictating microscope illumination minimized the confounding effects of surgeon variability. Surgeons were, likewise, able to perform the procedure with relative ease since the phacoemulsification time was within acceptable limits. Although a single surgeon series would have been ideal, we feel that the quality of the surgeries in this series came very close in terms of consistency. A larger sample size would have allowed us to analyze the results with a higher confidence level. Future studies can evaluate the diameter of the pupil when other types of acrylic intraocular lenses are used (e.g. accommodating IOLs, multifocal IOLs). In conclusion, topical nepafenac 0.1% has been shown to be a more effective inhibitor of miosis during phacoemulsification with IOL implantation compared with topical ketorolac or BSS.
Linebarger EJ, Hardten DR, Shah GK, et al. Phacoemulsification and modern cataract surgery. Surv Ophthalmol 1999; 44: 123-147.
Gogate PM, Kulkarni SR, Krishnaiah S, et al. Safety and efficacy of phacoemulsification compared with manual small-incision cataract surgery by a randomized controlled clinical trial: six-week results. Ophthalmology 2005;112: 869-874.
Riaz Y, Mehta JS, Wormald R, et al. Surgical interventions for age-related cataract. Cochrane Database Syst Rev 2006; 4: CD001323.
Gupta VP, Dhaliwal U, Prasad N. Ketorolac tromethamine in the maintenance of intraoperative mydriasis. Ophthalmic Surg Lasers 1997; 28: 731-738.
Solomon K, Turkalj JW, Whiteside SB. Topical 0.5% ketorolac vs 0.03% flurbiprofen for inhibition of miosis during cataract surgery. Arch Ophthalmol 1997; 115: 1119- 1122.
Cervantes-Coste G, Sanchez-Castro YG, Orozco-Carroll M, et al. Inhibition of surgically induced miosis and prevention of postoperative macular edema with nepafenac. Clin Ophthalmol 2009; 3: 219-226.
Duffin RM, Camras CB, Gardner SK, et al. Inhibitors of surgically induced miosis. Ophthalmology 1982; 89: 966-977.
Podos SM. Prostaglandins, nonsteroidal antiinflammatory agents and eye disease. Trans Am Ophthalmol Soc 1976; 74: 637-660.
Flach AJ, Lavelle CJ, Olander KW, et al. The effect of ketorolac tromethamine solution 0.5% in reducing postoperative inflammation after cataract extraction and intraocular-lens implantation. Ophthalmology 1988; 95: 1279-1284.
Ophthalmic Nonsteroidal Anti Inflammatory Drugs Drug Class Review. July 2009. http://vaww.pbm.va.gov (accessed Feb 22, 2010).
Ophthalmic NSAIDs Review. Sept 2008. https://nevada.fhsc.com/Downloads/ provider/NVRx_DCR_20090326_Ophthalmic_NSAIDs.pdf (accessed Oct 3, 2010).
Bucci FA Jr, Waterbury LD, Amico LM, et al. Prostaglandin E2 inhibition and aqueous concentration of ketorolac 0.4% (Acular LS) and nepafenac 0.1% (Nevanac) in patients undergoing phacoemulsification. Am J Ophthalmol 2007; 144: 146-147.
Acknowledgment The authors thank Ms. Kaice Cristobal for the statistical analyses.

